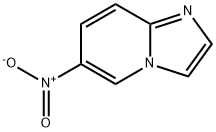
6-NITROIMIDAZO[1,2-A]PYRIDINE synthesis
- Product Name:6-NITROIMIDAZO[1,2-A]PYRIDINE
- CAS Number:25045-82-3
- Molecular formula:C7H5N3O2
- Molecular Weight:163.13
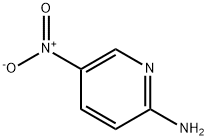
4214-76-0
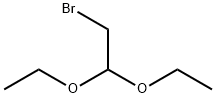
2032-35-1
![6-NITROIMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/25045-82-3.gif)
25045-82-3
In a 250 mL single-neck flask, bromoacetaldehyde diethyl acetal (42.5 g, 215.7 mmol) was dissolved in a mixture of 1 mol/L hydrochloric acid (120 mL) and ethanol (20 mL) and stirred at room temperature for 30 minutes. It was then heated to reflux and stirred continuously until the solution became clear. Upon completion of the reaction, the solution was cooled to room temperature and the pH was adjusted to near neutral with sodium bicarbonate solution. 2-Amino-5-nitropyridine (15.0 g, 107.8 mmol) was added and the reaction was stirred overnight at room temperature. At the end of the reaction, extraction was carried out with ethyl acetate (100 mL×3), the organic phases were combined, and the solvent was removed by concentration under reduced pressure. The crude product was purified by silica gel column chromatography (eluent ratio: petroleum ether:ethyl acetate = 2:1) to afford 13.7 g of yellow-brown crystalline 6-nitroimidazo[1,2-a]pyridine in 77.6% yield.

4214-76-0
522 suppliers
$6.00/10g

2032-35-1
488 suppliers
$5.00/10g
![6-NITROIMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/25045-82-3.gif)
25045-82-3
107 suppliers
$7.00/250mg
Yield:25045-82-3 96.7%
Reaction Conditions:
Stage #1: Bromoacetaldehyde diethyl acetalwith hydrogenchloride in water at 48; for 2 h;
Stage #2: 5-nitro-pyridin-2-ylaminewith sodium carbonate in water at 65; pH=8 - 9; for 2 h;
Steps:
2.2.1. Synthesis of N-(8-iodoimidazo[1,2-a]pyridin-6-yl)acetamide ( 6 )
H 2 O (100 mL), bromoacetaldehyde diethyl acetal (97%, 108.09 mL, 696.96 mmol), and hydrochloric acid (10 mL) were added to a clean round-bottom flask (250 mL). The mixture was heated to 48 °C, and then cooled to room temperature by stirring for 2 h. The pH was adjusted to 8-9 with solid Na 2 CO 3 in an ice water bath, and compound 2 (50 g, 359.43 mmol) was added. Next, the reaction mixture was heated to 65 °C for 2 h. The reactional time was monitored by TLC using mixture of petroleum ether and ethyl acetate (1:1) and the plates were revealed with sublimated iodine supported on silica gel. At the end of the reaction, the mixture was cooled to room temperature and the pH of the mixture was ad- justed to 9-10 by adding 10% NaOH solution. After the adjustment, a solid was precipitated out, and was then filtered and dried under vacuum to afford the yellow powder 3 (56.70 g, yield 96.70%).
References:
Chen, Dongmei;Chen, Yumei;Liao, Weike;Wu, Qingmei;Zhang, Xiaohan;Zhou, Zhixu [Journal of Molecular Structure,2021,vol. 1245]
107-20-0
0 suppliers
$22.60/5ml

4214-76-0
522 suppliers
$6.00/10g
![6-NITROIMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/25045-82-3.gif)
25045-82-3
107 suppliers
$7.00/250mg

4214-76-0
522 suppliers
$6.00/10g
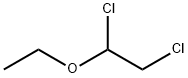
623-46-1
80 suppliers
$329.00/25g
![6-NITROIMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/25045-82-3.gif)
25045-82-3
107 suppliers
$7.00/250mg