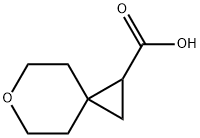
6-Oxaspiro[2.5]octane-1-carboxylic acid synthesis
- Product Name:6-Oxaspiro[2.5]octane-1-carboxylic acid
- CAS Number:909406-73-1
- Molecular formula:C8H12O3
- Molecular Weight:156.18
![Ethyl 6-oxaspiro[2.5]octane-1-carboxylate](/CAS/20150408/GIF/909406-74-2.gif)
909406-74-2
![6-Oxaspiro[2.5]octane-1-carboxylic acid](/CAS/20140602/GIF/CB82698447.gif)
909406-73-1
General procedure for the synthesis of 6-oxaspiro[2.5]octane-1-carboxylic acid from ethyl 6-oxaspiro[2.5]octane-1-carboxylate: to a solvent mixture (THF:MeOH:H2O = 2:1:1, total volume 6 mL) containing ester B-6a (50 mg, 0.271 mmol) was added LiOH (65.0 mg, 2.71 mmol). The reaction mixture was stirred at 0 °C and subsequently brought to room temperature and continued stirring for 12 hours. Upon completion of the reaction, the mixture was cooled to 0 °C and the pH was adjusted with 1.5 N HCl solution to about 3. Next, the reaction mixture was extracted with DCM (2 x 50 mL), the organic phases were combined and washed with brine (25 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure to afford the target product Cap B-6 (35 mg) as a colorless liquid. The product was characterized by 1H NMR (CDCl3, δ=7.26 ppm, 300 MHz): δ3.83-3.68 (m, 4H), 1.85-1.81 (m, 2H), 1.62-1.42 (m, 3H), 1.21 (t, J=6.4 Hz, 1H), 1.07-1.01 (m, 1H).
![Ethyl 6-oxaspiro[2.5]octane-1-carboxylate](/CAS/20150408/GIF/909406-74-2.gif)
909406-74-2
38 suppliers
$220.00/1g
![6-Oxaspiro[2.5]octane-1-carboxylic acid](/CAS/20140602/GIF/CB82698447.gif)
909406-73-1
50 suppliers
$60.00/50mg
Yield:909406-73-1 35 mg
Reaction Conditions:
with water;lithium hydroxide in tetrahydrofuran;methanol at 0 - 20; for 12 h;
Steps:
To a solution of ester B-6a (50 mg, 0.271 mmol) in (2: 1: 1) of THF (3 mL), MeOH (1.5 mL) and water (1.5 mL) was added LiOH (65.0 mg, 2.71 mmol) at 0 °C and stirred for 12 h at room temperature. Then the reaction mixture was cooled to 0 °C and adjusted the pH of the reaction mixture to (~3) with 1.5N HC1 solution. Then the reaction mixture was extracted with DCM (2x50 mL) and the organic later was washed with brine (25 mL), dried over Na2S04, filtered, and concentrated under reduced pressure to obtain Cap B-6 (35 mg) as a color less liquid.XH NMR (CDC13, δ = 7.26 ppm, 300 MHz): δ 3.83-3.68 (m, 4 H), 1.85-1.81 (m, 2 H), 1.62-1.42 (m, 3 H) 1.21 (t, J = 6.4, 1 H), 1.07-1.01 (m, 1 H).
References:
WO2015/5901,2015,A1 Location in patent:Page/Page column 625; 626
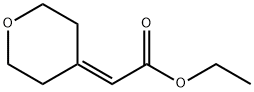
130312-00-4
85 suppliers
$40.00/250mg
![6-Oxaspiro[2.5]octane-1-carboxylic acid](/CAS/20140602/GIF/CB82698447.gif)
909406-73-1
50 suppliers
$60.00/50mg
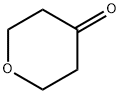
29943-42-8
433 suppliers
$5.00/1g
![6-Oxaspiro[2.5]octane-1-carboxylic acid](/CAS/20140602/GIF/CB82698447.gif)
909406-73-1
50 suppliers
$60.00/50mg