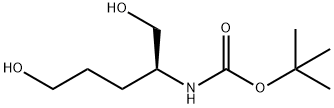
(S)-2-((tert-Butoxycarbonyl)amino)pentane-1,5-diyl Dimethanesulfonate synthesis
- Product Name:(S)-2-((tert-Butoxycarbonyl)amino)pentane-1,5-diyl Dimethanesulfonate
- CAS Number:607376-86-3
- Molecular formula:C12H25NO8S2
- Molecular Weight:375.46
Yield:607376-86-3 98%
Reaction Conditions:
with triethylamine in ethyl acetate at 0; for 2 h;
Steps:
(S)-2-(tert-Butoxycarbonylamino)pentane-1,5-diyl dimethanesulfonate (Intermediate 40):
A 250 mL roundbottom flask was charged with (S)-tert-butyl 1,5-dihydroxypentan-2-ylcarbamate (4.05 g, 18.47 mmol). EtOAc (75 mL) and triethylamine (6.20 mL, 44.5 mmol) were added, and the colorless solution was cooled to 0 °C. Methanesulfonyl chloride (3.00 mL, 38.5 mmol) was added dropwise, and the resulting colorless heterogeneous mixture was allowed to stir at 0 °C. After 2 hours, the reaction was partitioned between EtOAc and H2O. The aqueous layer was extracted with EtOAc, and the combined organics were washed with brine, dried (Na2SO4), filtered, and concentrated under reduced pressure to give the product as a colorless solid (6.81 g, 98%).
References:
Hennessy, Edward J.;Saeh, Jamal C.;Sha, Li;MacIntyre, Terry;Wang, Haiyun;Larsen, Nicholas A.;Aquila, Brian M.;Ferguson, Andrew D.;Laing, Naomi M.;Omer, Charles A. [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 4,p. 1690 - 1694] Location in patent:supporting information; experimental part
56-86-0
867 suppliers
$5.00/25g

607376-86-3
6 suppliers
inquiry
23150-65-4
334 suppliers
$6.00/10g

607376-86-3
6 suppliers
inquiry