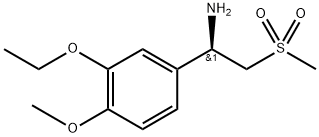
(R)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine synthesis
- Product Name:(R)-1-(3-Ethoxy-4-Methoxyphenyl)-2-(Methylsulfonyl)ethylaMine
- CAS Number:608142-27-4
- Molecular formula:C12H19NO4S
- Molecular Weight:273.35
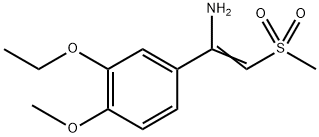
1450657-31-4
22 suppliers
inquiry

608142-27-4
54 suppliers
inquiry
Yield: 70%
Reaction Conditions:
with bis(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate;(2R)-1-[(1R)-1-[bis(1,1-dimethylethyl)phosphino]ethyl]-2-(diphenylphosphino)ferrocene;hydrogen in 2,2,2-trifluoroethanol at 50; under 5414.51 Torr; for 18 h;Inert atmosphere;Reagent/catalyst;Pressure;
Steps:
2 EXAMPLE 2 Synthesis of (R)-1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethanamine
A solution of bis(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate (36 mg, 0.074 mmol) and (R)-1-[(S)-2-(diphenylphosphino)ferrocenyl]ethyldi-tert-butylphosphine (40 mg, 0.074 mmol) in 25 mL of 2,2,2-trifluoroethanol was prepared under nitrogen. To this solution was then charged 1-(3-ethoxy-4-methoxyphenyl)-2-(methylsulfonyl)ethenamine (2.0 g, 7.4 mmol). The resulting mixture was heated to 50° C. and hydrogenated under 90 psig hydrogen pressure. After 18 h, the mixture was cooled to ambient temperature and removed from the hydrogenator. The mixture was evaporated and the residue was purified by chromatography on a C18 reverse phase column using a water-acetonitrile gradient. The appropriate fractions were pooled and evaporated to 150 mL. To this solution was added brine (20 mL), and the resulting solution was extracted with EtOAc (3×50 mL). The combined organic layers were dried (MgSO4) and evaporated to provide the product as a white crystalline solid (1.4 g, 70% yield); achiral HPLC (Hypersil BDS C8, 5.0 μm, 250×4.6 mm, 1.5 mL/min, 278 nm, 90/10 gradient to 80/20 0.1% aqueous TFA/MeOH over 10 min then gradient to 10/90 0.1% aqueous TFA/MeOH over the next 15 min): 9.11 (99.6%); chiral HPLC (Chiralpak AD-H 5.0 μm Daicel, 250×4.6 mm, 1.0 mL/min, 280 nm, 70:30:0.1 heptane-i-PrOH-diethylamine): 7.32 (97.5%), 8.26 (2.47%); 1H NMR (DMSO-d6) δ 1.32 (t, J=7.0 Hz, 3H), 2.08 (s, 2H), 2.96 (s, 3H), 3.23 (dd, J=3.6, 14.4 Hz, 1H), 3.41 (dd, J=9.4, 14.4 Hz, 1H), 3.73 (s, 3H), 4.02 (q, J=7.0 Hz, 2H), 4.26 (dd, J=3.7, 9.3 Hz, 1H), 6.89 (s, 2H), 7.02 (s, 1H); 13C NMR (DMSO-d6) δ 14.77, 41.98, 50.89, 55.54, 62.03, 63.68, 111.48, 111.77, 118.36, 137.30, 147.93, 148.09.
References:
CELGENE CORPORATION;CONNOLLY, Terrence J.;RUCHELMAN, Alexander L.;LEONG, William W. US2013/217919, 2013, A1 Location in patent:Paragraph 0276-0277

1450657-31-4
22 suppliers
inquiry
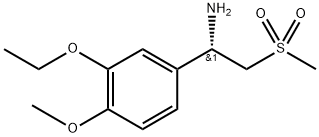
608141-42-0
223 suppliers
$10.00/250mg

608142-27-4
54 suppliers
inquiry
608142-28-5
6 suppliers
inquiry

608142-27-4
54 suppliers
inquiry
![3-Ethoxy-4-Methoxy-alpha-[(Methylsulfonyl)Methyl]-benzeneMethanaMine](/CAS/20150408/GIF/253168-94-4.gif)
253168-94-4
247 suppliers
$7.00/250mg

608142-27-4
54 suppliers
inquiry
![3-Ethoxy-4-Methoxy-alpha-[(Methylsulfonyl)Methyl]-benzeneMethanaMine](/CAS/20150408/GIF/253168-94-4.gif)
253168-94-4
247 suppliers
$7.00/250mg

608141-42-0
223 suppliers
$10.00/250mg

608142-27-4
54 suppliers
inquiry