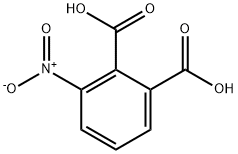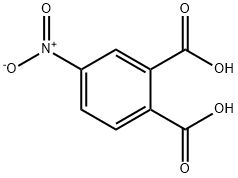
4-Nitrophthalic acid synthesis
- Product Name:4-Nitrophthalic acid
- CAS Number:610-27-5
- Molecular formula:C8H5NO6
- Molecular Weight:211.13
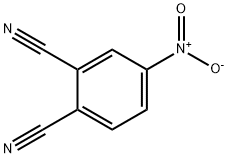
31643-49-9

610-27-5
The general procedure for the synthesis of 4-nitrophthalic acid from 4-nitrophthalonitrile was as follows: 4-nitrophthalonitrile (2 mmol) was dissolved in 5 ml of [bmim]HSO4 ionic liquid and the reaction mixture was heated at 60-65°C for 1-3 hours. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the mixture was poured into water containing crushed ice to induce precipitation of the product. Subsequently, the precipitate is collected by filtration and dried. The product yield of this method was generally higher than 90% and the purity of the product met the requirements without further purification. The filtrate was concentrated under vacuum, washed twice with diethyl ether and concentrated again under high vacuum. After drying, about 95% of the ionic liquid was recovered, and its authenticity was verified by comparison with the original ionic liquid. The recovered ionic liquid showed comparable efficiency to the original ionic liquid in the reaction of converting nitrile to acid and could be reused 5-6 times without significant reduction in activity.

31643-49-9
466 suppliers
$6.00/5g

610-27-5
441 suppliers
$6.00/5g
Yield:610-27-5 95%
Reaction Conditions:
with 1-butyl-3-methylimidazolium hydrogen sulfate;water at 60 - 65; for 2.45 h;Green chemistry;
Steps:
General procedure for the synthesis of carboxylic acids from nitriles
General procedure: Aromatic or aliphatic nitriles (2 mmol) were dissolved in 5 ml of [bmim]HSO4 and the reaction mixture was heated at 60-65 °C for 1-3 h. The progress of reaction was monitored by TLC. After completion of reaction, as checked by TLC, the reaction mixture was poured into water containing crushed ice. The product was precipitated out, filtered and dried. The yield of the final product was high (>90%) in all cases. All final products obtained were found sufficiently pure so it didn’t need further purification.The filtrate was concentrated under vacuum, washed with diethylether twice and concentrated under high vacuum. After proper drying under reduced pressure, approximately 95% ionic liquid was recovered from the reaction and compared with the original ionic liquid to check its authenticity. The efficiency of recovered ionic liquid in conversion of nitriles to acids was found unchanged in comparison to the original one and we reused it up to 5-6 cycles without any significant loss of its activity.
References:
Kumar, Satyanand;Dixit, Sandeep Kumar;Awasthi, Satish Kumar [Tetrahedron Letters,2014,vol. 55,# 28,p. 3802 - 3804] Location in patent:supporting information
99-51-4
26 suppliers
$13.00/25g

610-27-5
441 suppliers
$6.00/5g
581-89-5
12 suppliers
$55.00/250mg

610-27-5
441 suppliers
$6.00/5g
88-99-3
479 suppliers
$15.00/50 g
85-44-9
780 suppliers
$9.00/5g

610-27-5
441 suppliers
$6.00/5g