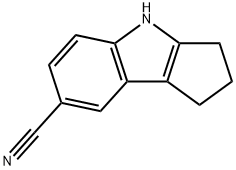
CYCLOPENT[B]INDOLE-7-CARBONITRILE, 1,2,3,4-TETRAHYDRO- synthesis
- Product Name:CYCLOPENT[B]INDOLE-7-CARBONITRILE, 1,2,3,4-TETRAHYDRO-
- CAS Number:628294-80-4
- Molecular formula:C12H10N2
- Molecular Weight:182.22
Yield:-
Reaction Conditions:
with sodium acetate;acetic acid for 2 h;Heating / reflux;
Steps:
10A
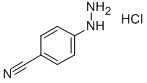
Example [10A] : 5-Cyanocyclopent [b] indole A solution of cyclopentanone (1 g, 12.0 mmol), 4-cyanophenylhydrazine hydrochloride [(2. 2] g, 13.1 mmol) and sodium acetate (1.07 g, 13 mmol) in glacial acetic acid (20 [ML)] was refluxed 2 hours then cooled and poured into water and extracted with dichloromethane. The organic layer was washed with [NAHCO3] sat. and water, then dried over sodium sulfate and solvent removed in vacuo. The crude was purified by column chromatography [(40/60] ethyl acetate/hexane) to give 980 mg of title compound. [C12HL2N2] Mass (calculated): [[182]] ; (found): [[M+H+]] = 183. NMR (400 MHz, [CDC13)] : 2.45-2. 55 (2H, m, cyclopentyl-H); 2.75-2. 85 (4H, m, cyclopentyl-H); 7.3 (2H, s, aryl-H); 7.8 [(1H,] s, aryl-H); 8.1 [(1H,] bs, NH).
References:
WO2003/99814,2003,A1 Location in patent:Page 27-28