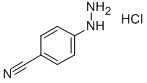
4-Cyanophenylhydrazine hydrochloride synthesis
- Product Name:4-Cyanophenylhydrazine hydrochloride
- CAS Number:2863-98-1
- Molecular formula:C7H8ClN3
- Molecular Weight:169.61
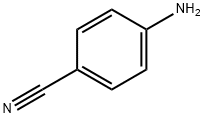
873-74-5

2863-98-1
The general procedure for the synthesis of 4-cyanophenylhydrazine hydrochloride from 4-aminobenzonitrile was as follows: to a stirring suspension of 4-aminobenzonitrile (50 g, 423 mmol) concentrated hydrochloric acid (550 mL) was slowly added dropwise an aqueous sodium nitrite solution (31.5 g, 457 mmol, dissolved in 200 mL of water) at a low temperature of -5 to 0 °C. Keeping the reaction temperature below 0 °C, a solution of tin(II) chloride dihydrate (477 g, 2.1 mol) concentrated hydrochloric acid (370 mL) pre-cooled to 0 °C was added to the above diazotized solution. The reaction mixture was continued to be stirred for 15 minutes. Upon completion of the reaction, the resulting white precipitate was collected by filtration, washed with diethyl ether and recrystallized from aqueous ethanol. The resulting 4-cyanophenylhydrazine hydrochloride had a melting point of 234-236 °C (literature value 235-237 °C) and yielded 60 g in 84% yield.

873-74-5
657 suppliers
$9.00/1g

2863-98-1
292 suppliers
$6.00/1g
Yield:2863-98-1 84%
Reaction Conditions:
with hydrogenchloride;tin(II) chloride dihdyrate;sodium nitrite in water at -5 - 0; for 0.25 h;
Steps:
1 4.1 Synthesis of 4-hydrazinylbenzonitrile hydrochloride (4)
To a cooled (-5 to 0°C) stirred suspension of 4-aminobenzonitrile (50g, 423mmol) in concentrated hydrochloric acid (550mL) was added dropwise aqueous sodium nitrite solution (31.5g, 457mmol in 200mL water). To this diazotised solution cooled (0°C) solution of tin (II) chloride dihydrate (477g, 2.1mol) in concentrated hydrochloric acid (370mL) was added while stirring and maintaining the temperature below 0°C. The resulting solution was further stirred for 15min. White precipitates so formed were collected by filtration, washed with diethylether and crystallized from aqueous ethanol. Mp 234-236°C (Lit. mp 235-237°C), yield 60g (84%).
References:
Chandna, Nisha;Kumar, Satish;Kaushik, Pawan;Kaushik, Dhirender;Roy, Somendu K.;Gupta, Girish K.;Jachak, Sanjay M.;Kapoor, Jitander K.;Sharma, Pawan K. [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 15,p. 4581 - 4590]
25102-85-6
0 suppliers
inquiry

2863-98-1
292 suppliers
$6.00/1g
3058-39-7
283 suppliers
$10.00/1g

2863-98-1
292 suppliers
$6.00/1g
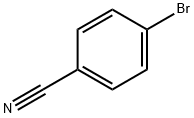
623-00-7
489 suppliers
$6.00/10g

2863-98-1
292 suppliers
$6.00/1g
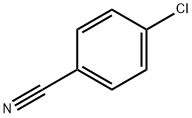
623-03-0
481 suppliers
$6.00/25g

2863-98-1
292 suppliers
$6.00/1g