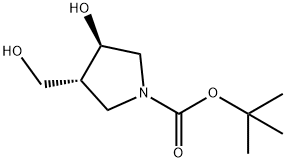
(3R,4R)-tert-Butyl 3-Hydroxy-4-(hydroxyMethyl)pyrrolidine-1-carboxylate synthesis
- Product Name:(3R,4R)-tert-Butyl 3-Hydroxy-4-(hydroxyMethyl)pyrrolidine-1-carboxylate
- CAS Number:635319-09-4
- Molecular formula:C10H19NO4
- Molecular Weight:217.26
![1-Pyrrolidinecarboxylic acid, 3-[(1S)-1,2-dihydroxyethyl]-4-hydroxy-, 1,1-dimethylethyl ester, (3S,4R)-](/CAS/20210305/GIF/635319-08-3.gif)
635319-08-3

635319-09-4
The general procedure for the synthesis of tert-butyl (3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidine-1-carboxylate from the compound (CAS: 635319-08-3) was as follows: to an ethanol ((S)-1,2-dihydroxyethyl)pyrrolidine (3.4 g, 13.7 mmol) solution of N-tert-butoxycarbonyl-(3R,4S)-3-hydroxy-4-[(S)-1,2-dihydroxyethyl]pyrrolidine ( 50 mL) solution of sodium periodate (3.4 g, 16 mmol) was added dropwise to an aqueous (25 mL) solution of sodium periodate (3.4 g, 16 mmol) while maintaining the reaction temperature at 0 °C. The reaction mixture was continued to be stirred at 0 °C for 20 min. Subsequently, sodium borohydride (2.0 g, excess) was added in batches to ensure that the reaction temperature was maintained at 0 °C. After completion of addition, the solids were removed by filtration and the filter cake was washed with ethanol (50 mL). The filtrates were combined and concentrated in vacuum to give a syrupy product. Purification by column chromatography gave the target product 1 (2.74 g, 92% yield) in syrupy form.
253129-03-2
47 suppliers
$50.00/10mg

24424-99-5
870 suppliers
$13.50/25G

635319-09-4
36 suppliers
$156.00/50mg
Yield:635319-09-4 100%
Reaction Conditions:
with hydrogen;palladium on activated charcoal in methanol; for 24 h;
References:
Clinch, Keith;Evans, Gary B.;Fleet, George W. J.;Furneaux, Richard H.;Johnson, Stephen W.;Lenz, Dirk H.;Mee, Simon P. H.;Rands, Peter R.;Schramm, Vern L.;Taylor Ringia, Erika A.;Tyler, Peter C. [Organic and Biomolecular Chemistry,2006,vol. 4,# 6,p. 1131 - 1139]
![1-Pyrrolidinecarboxylic acid, 3-[(1S)-1,2-dihydroxyethyl]-4-hydroxy-, 1,1-dimethylethyl ester, (3S,4R)-](/CAS/20210305/GIF/635319-08-3.gif)
635319-08-3
0 suppliers
inquiry

635319-09-4
36 suppliers
$156.00/50mg

24424-99-5
870 suppliers
$13.50/25G

267421-93-2
11 suppliers
inquiry

635319-09-4
36 suppliers
$156.00/50mg

849935-77-9
1 suppliers
inquiry

635319-09-4
36 suppliers
$156.00/50mg

24424-99-5
870 suppliers
$13.50/25G

635319-09-4
36 suppliers
$156.00/50mg