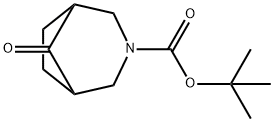
tert-butyl 8-oxo-3-azabicyclo[3.2.1]octane-3-carboxylate synthesis
- Product Name:tert-butyl 8-oxo-3-azabicyclo[3.2.1]octane-3-carboxylate
- CAS Number:637301-19-0
- Molecular formula:C12H19NO3
- Molecular Weight:225.28

24424-99-5
![3-BENZYL-3-AZABICYCLO[3.2.1]OCTAN-8-ONE](/CAS/GIF/83507-33-9.gif)
83507-33-9
![tert-butyl 8-oxo-3-azabicyclo[3.2.1]octane-3-carboxylate](/CAS/GIF/637301-19-0.gif)
637301-19-0
Di-tert-butyl dicarbonate (5.81 g, 26.6 mmol) and 3-benzyl-3-azabicyclo[3.2.1]octan-8-one (5.0 g, 22.0 mmol) were used as raw materials in ethyl acetate (74 mL) with Pearlman's catalyst (1.55 g, 23.2 mmol). The reaction system was stirred overnight at 25 °C under 100 psi hydrogen pressure after passing through three nitrogen substitutions and two hydrogen substitutions. After completion of the reaction, the mixture was filtered through a diatomaceous earth pad and washed with ethyl acetate. The filtrate was concentrated and purified by silica gel column chromatography (elution gradient: heptane/ethyl acetate, 0:100 to 100:0) to afford 3-Boc-3-azabicyclo[3.2.1]octan-8-one (4.49 g, 90% yield) as a solid. The product was analyzed by LC/MS showing [M-Me] = 211.1; 1H NMR (400 MHz, CDCl3) δ 4.38 (d, J = 14.0 Hz, 1H), 4.20 (d, J = 14.0 Hz, 1H), 3.27 (d, J = 13.3 Hz, 1H), 3.17 (d, J = 13.3 Hz, 1H), 2.24 ( d, J = 15.6 Hz, 2H), 1.76-1.99 (m, 4H), 1.49 (s, 9H).

24424-99-5
871 suppliers
$13.50/25G
![3-BENZYL-3-AZABICYCLO[3.2.1]OCTAN-8-ONE](/CAS/GIF/83507-33-9.gif)
83507-33-9
160 suppliers
$16.00/100mg
![tert-butyl 8-oxo-3-azabicyclo[3.2.1]octane-3-carboxylate](/CAS/GIF/637301-19-0.gif)
637301-19-0
94 suppliers
$45.00/10mg
Yield: 90%
Reaction Conditions:
with 10 wt% Pd(OH)2 on carbon;hydrogen in ethyl acetate at 25; under 5171.62 Torr;
Steps:
20.1 Step 1 : tert-butyl 8-oxo-3-azabicyclo[3.2.1]octane-3-carboxylate
Di-tert-butyl dicarbonate (5.81 g, 26.6 mmol) and Pearlman's catalyst (1.55 g, 23.2 mmol) were successively added to a solution of 3-benzyl-3-azabicyclo[3.2.1]octan-8-one obtained from commercial sources (Reference Mityuk, et al, Synthesis, 2010, 493-97, CAS 83507-33-9) (5.0 g, 22.0 mmol) in EtOAc (74 mL) at 25 °C. The reaction vessel was alternately filled with nitrogen and evacuated (3x) and then filled and evacuated with hydrogen (2x). The mixture was stirred overnight under 100 psi of H2. The mixture was filtered through a pad of Celite which was washed with ethyl acetate. The filtrate was concentrated and the crude product was purified by silica gel chromatography (heptane: EtOAc, 0: 100-100:0) to provide the title compound (4.49 g, 90%) as a solid. LC/MS [M-Me] = 21 1.1 ; 1H NMR (400 MHz, CDCI3) δ 4.38 (d, J=14.0 Hz, 1 H), 4.20 (d, J=14.0 Hz, 1 H), 3.27 (d, J=13.3 Hz, 1 H), 3.17 (d, J=13.3 Hz, 1 H), 2.24 (d, J=15.6 Hz, 2H), 1.76-1.99 (m, 4H), 1.49 (s, 9H).
References:
PFIZER INC.;SCHNUTE, Mark Edward;FLICK, Andrew Christopher;JONES, Peter;KAILA, Neelu;MENTE, Scot Richard;TRZUPEK, John David;VAZQUEZ, Michael L.;XING, Li;ZHANG, Liying;WENNERSTAL, Goran Mattias;ZAMARATSKI, Edouard WO2016/120849, 2016, A1 Location in patent:Page/Page column 63

120-92-3
427 suppliers
$11.00/25g
![tert-butyl 8-oxo-3-azabicyclo[3.2.1]octane-3-carboxylate](/CAS/GIF/637301-19-0.gif)
637301-19-0
94 suppliers
$45.00/10mg