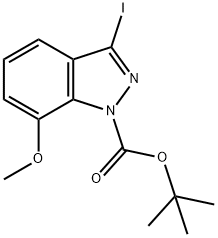
tert-Butyl 3-iodo-7-methoxy-1H-indazole-1-carboxylate synthesis
- Product Name:tert-Butyl 3-iodo-7-methoxy-1H-indazole-1-carboxylate
- CAS Number:639084-05-2
- Molecular formula:C13H15IN2O3
- Molecular Weight:374.17

24424-99-5
864 suppliers
$13.50/25G
351210-07-6
58 suppliers
$57.00/100mg

639084-05-2
25 suppliers
inquiry
Yield:639084-05-2 98%
Reaction Conditions:
with dmap;triethylamine in acetonitrile at 20; for 3 h;
Steps:
116 tert-Butyl 3-iodo-7-methoxy-1H-indazole-1-carboxylate
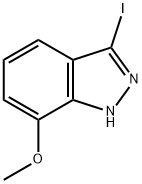
To a solution of 3-iodo-7-methoxy-1H-indazole (1.33 g, 4.86 mmol) in acetonitrile (20 mL) are added di-t-butyl bicarbonate (1.34 mL, 5.83 mmol), triethylamine (0.81 mL, 5.81 mmol) and N,N-dimethylaminopyridine (60.0 mg, 0.491 mmol), and the mixture is stirred at room temperature for 3 hours. To the reaction solution is added water, and the mixture is extracted with ethyl acetate. The organic layer is washed with a saturated brine, and dried over anhydrous magnesium sulfate. The solvent is evaporated under reduced pressure, and the obtained residue is purified by silica gel column chromatography (n-hexane/ethyl acetate = 5/1) to give the title compound (1.79 g, yield: 98 %). 1H-NMR (CDCl3) δ: 1.68 (9H, s), 3.99 (3H, s), 7.01 (1H, d, J=7.8Hz), 7.10 (1H, dd, J=8.0, 0.7Hz), 7.30 (1H, dd, J=8.0, 7.8Hz).
References:
EP1514869,2005,A1 Location in patent:Page/Page column 83-84