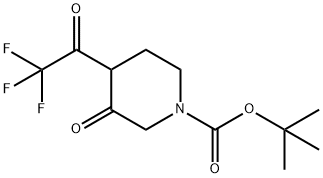
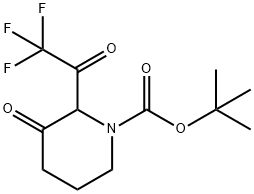
tert-Butyl 3-oxo-4-(2,2,2-trifluoroacetyl)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 3-oxo-4-(2,2,2-trifluoroacetyl)piperidine-1-carboxylate
- CAS Number:647863-25-0
- Molecular formula:C12H16F3NO4
- Molecular Weight:295.25
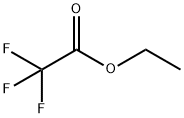
383-63-1
498 suppliers
$10.00/25g
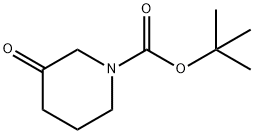
98977-36-7
409 suppliers
$6.00/1g

647863-25-0
35 suppliers
$290.00/250mg
Yield:647863-25-0 81%
Reaction Conditions:
Stage #1: 3-oxo-piperidine-1-carboxylic acid tert-butyl esterwith lithium hexamethyldisilazane in tetrahydrofuran;1,2-dimethoxyethane at -78; for 1 h;Cooling with acetone-dry ice;
Stage #2: ethyl trifluoroacetate, in tetrahydrofuran;1,2-dimethoxyethane at -78 - 20; for 3.5 h;
Steps:
3.a; 47
5.0 g (25 mmol) of t-butyl 3-oxpiperidin-l -carboxylate was dissolved in dimethoxyethane, and the resulting solution was cooled to -78°C, then 30 mL (30 mmol) of lithium hexamethyldisilazane (LHMDS, IM in THF) was dropwise added and stirred for about 1 hour, followed by dropwise addition of 3.9 mL (33 mmol) of ethyltrifluoroacetate. After stirring for 1 hour, a dryice/acetone bath was removed and then further stirred for about 2 hours and 30 minutes with the reaction solution being heated to room temperature. After the reaction solution was washed with a saturated aqueous ammonium chloride, extraction was conduced three times with ethyl acetate. An organic layer was dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure, then the residue was purified by column chromatography (20:1 dichloromethane:methanol) to give 6.0 g of the title compound in a yield of 81%.[831] 1K NMR (CDCl3) δ 4.22 (2H, br s), 3.56 (2H, m), 2.57 (2H, br s), 1.49 (9H, s)[832] Mass (m/e) 296 (M+l)
References:
WO2006/104356,2006,A1 Location in patent:Page/Page column 18; 61

98977-36-7
409 suppliers
$6.00/1g

647863-25-0
35 suppliers
$290.00/250mg
![1(2H)-Pyridinecarboxylic acid, 3,4-dihydro-5-[(trimethylsilyl)oxy]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/647863-23-8.gif)
647863-23-8
0 suppliers
inquiry
![1(2H)-Pyridinecarboxylic acid, 5,6-dihydro-3-[(trimethylsilyl)oxy]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/647863-24-9.gif)
647863-24-9
1 suppliers
inquiry
407-25-0
470 suppliers
$9.00/1g

647863-25-0
35 suppliers
$290.00/250mg
647863-26-1
0 suppliers
inquiry