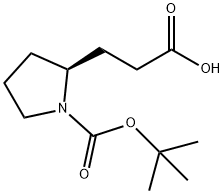
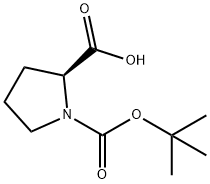
3-[(2S)-1-[(tert-butoxy)carbonyl]pyrrolidin-2-yl]propanoic acid synthesis
- Product Name:3-[(2S)-1-[(tert-butoxy)carbonyl]pyrrolidin-2-yl]propanoic acid
- CAS Number:65595-02-0
- Molecular formula:C12H21NO4
- Molecular Weight:243.3
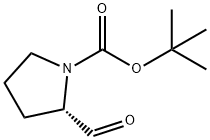
741269-02-3
7 suppliers
inquiry
![3-[(2S)-1-[(tert-butoxy)carbonyl]pyrrolidin-2-yl]propanoic acid](/CAS/20200331/GIF/65595-02-0.gif)
65595-02-0
12 suppliers
inquiry
Yield:65595-02-0 98%
Reaction Conditions:
with hydrogen;palladium on activated carbon in ethanol; for 24 h;
Steps:
2
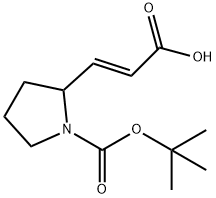
Reference Example 2; A mixture of ethyl (2E)-3-[1-(tert-butoxycarbonyl)pyrrolidin-2-yl]-2-propenoate (5.31 g) and 10% Pd-C (0.50 g) in ethanol was stirred under a hydrogen atmosphere for 24 hours. After removing the catalyst by filtration, the resulting residue was concentrated under reduced pressure to give 3-[1-(tert-butoxycarbonyl)pyrrolidin-2-yl]-2-propionic acid (5.32 g, 98%) as a colorless oily material. To a solution of 3-[1-(tert-butoxycarbonyl)pyrrolidin-2-yl]propionic acid (5.32 g) in ethanol (50 ml) was added concentrated hydrochloric acid (50 ml), and the mixture was stirred at room temperature for 20 hours. After concentration under reduced pressure, to the residue were added water (25 ml) and concentrated hydrochloric acid (50 ml), and the mixture was stirred at 120°C for 20 hours. After concentration under reduced pressure, 2-propanol was added thereto and the mixture was concentrated. To the residue were added 2-propanol and diisopropyl ether. The precipitated crystals were collected by filtration. The crystals were washed with diisopropyl ether to give 3-(pyrrolidin-2-yl)propionic acid hydrochloride (1.91 g) as colorless crystals. 1H-NMR (200MHz, DMSO-d6) δ 1.43-1.67 (2H, m), 1.66-2.14 (4H, m), 2.37 (2H, t, J=7.5 Hz), 3.00-3.21 (2H, m), 3.29-3.50 (1H, m), 8.62-8.88 (1H, m), 9.24-9.28 (1H, m). IR (KBr) 3113, 1732, 1705, 1599, 1420, 1206, 1173, 878 cm-1
References:
EP1593681,2005,A1 Location in patent:Page/Page column 41-42
15761-39-4
619 suppliers
$5.00/10g
79-10-7
728 suppliers
$20.50/500ml
![3-[(2S)-1-[(tert-butoxy)carbonyl]pyrrolidin-2-yl]propanoic acid](/CAS/20200331/GIF/65595-02-0.gif)
65595-02-0
12 suppliers
inquiry