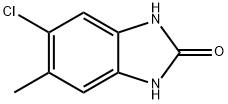
2H-Benzimidazol-2-one,5-chloro-1,3-dihydro-6-methyl-(9CI) synthesis
- Product Name:2H-Benzimidazol-2-one,5-chloro-1,3-dihydro-6-methyl-(9CI)
- CAS Number:683240-81-5
- Molecular formula:C8H7ClN2O
- Molecular Weight:182.61
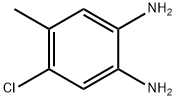
63155-04-4
86 suppliers
$38.00/1g
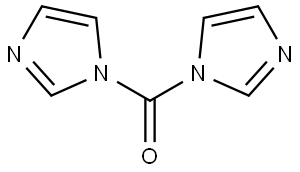
530-62-1
969 suppliers
$6.00/25g

683240-81-5
25 suppliers
$225.00/1g
Yield:683240-81-5 80%
Reaction Conditions:
in tetrahydrofuran at 20; for 3 h;Inert atmosphere;
Steps:
49.a
Example 49: l-(5-chloro-l-cyclobutyl-6-methyl-lH-benzo[d]imidazol-2-yl)-7V-cyclopentyl- piperidine-4-carboxamide and l-(6-chloro-l-cyclobutyl-5-methyl-lH-benzo[d]imidazol-2- yl)-7V-cyclopentylpiperidine-4-carboxamide(a) Preparation of the intermediate compound 5-chloro-6-methyl-lH-benzo[d]imidazol-2(3Η)- one:To a stirred solution of 4-chloro-5-methylbenzene-l,2-diamine (0.3 g, 1.9 mmol) intetrahydrofuran (5 mL) was added JV,iV-carbonyl dimidazole (CDI, 0.465 g, 2.8 mmol). The reaction mixture was stirred at room temperature for 3 hours under Argon gas, concentrated in vacuo and the residue partitioned between ethyl acetate (50 mL) and aqueous sodium hydroxide (50 mL, IN). The aqueous extract was acidified to pH 5 via addition of aqueous hydrochloric acid (1.57V) and the resulting brown precipitate was filtered, washed with water (3χ20mL) and dried under high vacuum to obtain 0.28 g (80 % yield) of 5-chloro-6-methyl-lH- benzo[d]imidazol-2(3H)-one.
References:
WO2011/23812,2011,A1 Location in patent:Page/Page column 95