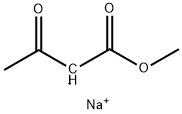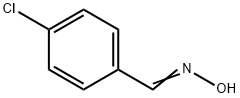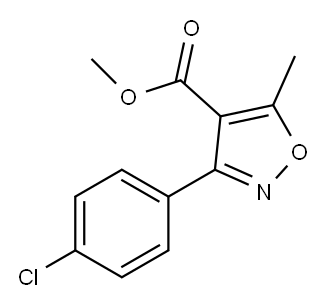
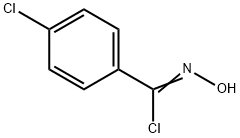
4-Isoxazolecarboxylic acid, 3-(4-chlorophenyl)-5-Methyl-, Methyl synthesis
- Product Name:4-Isoxazolecarboxylic acid, 3-(4-chlorophenyl)-5-Methyl-, Methyl
- CAS Number:68870-58-6
- Molecular formula:C12H10ClNO3
- Molecular Weight:251.67
Yield: 86%
Reaction Conditions:
in neat (no solvent) at 20; for 0.333333 h;Milling;Green chemistry;regioselective reaction;
Steps:
1 4.2. General procedure for the synthesis of isoxazoles from N-hydroxybenzimidoyl chlorides and enamino carbonyl compounds
General procedure: A mixture of a N-hydroxybenzimidoyl chloride 1 (0.22 mmol) and an enamino carbonyl compound 2 (0.2 mmol) was added to a stainless-steel jar (5 mL, 12 mm x 50 mm, 61.556 g) containing two stainless-steel balls (2 x 0.519 g, 5 mm in diameter). The mass ratio of the reaction mixture to balls were from 1:12.5 to 1:15.8. The same mixture was also added to a second parallel jar. The two reaction vessels were vibrated in Spex SamplePrep 8000 mixer mill at a frequency of 875 cycles per minute at room temperature for 20-60 min. The combined reaction mixtures from two vessels were collected and dissolved in ethyl acetate. The solution was filtrated to remove the residue and then evaporated to dryness under reduced pressure. Finally, the resulting product 3 was purified by column chromatography over silica gel using ethyl acetate/petroleum ether as the eluent. 4.2.1. Methyl 3-(4-chlorophenyl)-5-methylisoxazole-4-carboxylate (3a) Yield 86% (86.7mg). 1H NMR (400MHz, CDCl3) δ 7.58 (d, J=8.5Hz, 2H), 7.42 (d, J=8.5Hz, 2H), 3.79 (s, 3H), 2.73 (s, 3H); 13C NMR (100MHz, CDCl3) δ 176.2, 162.3, 161.7, 136.1, 130.7, 128.4, 126.9, 108.2, 51.7, 13.7; HRMS (EI-TOF) m/z [M]+ calcd for C12H1035ClNO3, 251.0349; found, 251.0346.
References:
Xu, Hui;Fan, Guang-Peng;Liu, Zi;Wang, Guan-Wu [Tetrahedron,2018,vol. 74,# 45,p. 6607 - 6611]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
28123-63-9
43 suppliers
$90.00/250 mg

68870-58-6
8 suppliers
inquiry
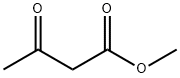
105-45-3
694 suppliers
$5.00/5g

28123-63-9
43 suppliers
$90.00/250 mg

68870-58-6
8 suppliers
inquiry

18913-35-4
18 suppliers
inquiry

28123-63-9
43 suppliers
$90.00/250 mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

68870-58-6
8 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![methyl 3-[(propan-2-yl)amino]but-2-enoate](/CAS/20200515/GIF/73967-70-1.gif)