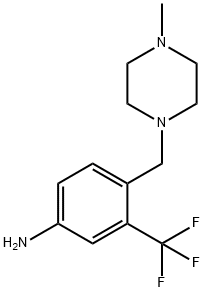
4-(4-Methylpiperazinomethyl)-3-(trifluoromethyl)aniline synthesis
- Product Name:4-(4-Methylpiperazinomethyl)-3-(trifluoromethyl)aniline
- CAS Number:694499-26-8
- Molecular formula:C13H18F3N3
- Molecular Weight:273.3

4-((4-Methylpiperazin-1-yl)methyl)-3-(trifluoromethyl)aniline Into a solution 4-methyl-1-(4-nitro-2-(trifluoromethyl)benzyl)piperazine (1.5 g, 5 mmol) in MeOH (250 mL) was added Raney Nickel (0.15 g, 10 wt %). The suspension was stirred under hydrogen atmosphere (50 psi) for 24 hrs and monitored by TLC. The reaction mixture was filtered through celite and the filtrate was concentrated under reduced pressure to yield the desired product (1.36 g, 100%). 1H NMR (300 MHz, CDCl3) δ: 7.43-7.46 (1H, d, J=9.0 Hz), 6.91 (1H, s), 6.77-6.80 (1H, d J=9.0 Hz), 3.77 (2H, s), 3.54 (2H, s), 2.53 (8H, brs), 2.34 (3H, s).
![Acetamide, 2,2,2-trifluoro-N-[4-[(4-methyl-1-piperazinyl)methyl]-3-(trifluoromethyl)phenyl]-](/CAS/20210305/GIF/709045-30-7.gif)
709045-30-7
0 suppliers
inquiry

694499-26-8
231 suppliers
$9.00/250mg
Yield:-
Reaction Conditions:
with potassium carbonate in methanol;water; for 1 h;
Steps:
33.4 Step 33.4 : [4- (4-METHYL-PIPERAZIN-1-YLMETHYL)-3-TRIFLUOROMETHYL] benzenamine
To a solution of 1.102 g (2.98 MMOL) of 2,2, 2-TRIFLUORO-N- [4- (4-METHYL-PIPERAZIN-1-YLMETHYL)-3- trifluoromethyl-phenyl]-acetamide in 26 ML of boiling methanol, 14 ML of a 1 M solution of K2CO3 in water are added dropwise. After 1 h stirring, the reaction mixture is cooled to rt and diluted with EtOAc and water. The aqueous layer is separated off and extracted twice with EtOAc. The organic phases are washed with water and brine, dried (NA2SO4) and concentrated to yield the title compound, which was directly used in Step 33.5 : MS: [M+1] +-274.
References:
WO2004/52884,2004,A1 Location in patent:Page 54
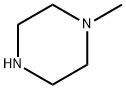
109-01-3
672 suppliers
$5.00/5G
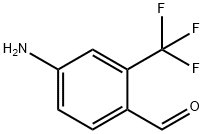
876322-73-5
17 suppliers
inquiry

694499-26-8
231 suppliers
$9.00/250mg
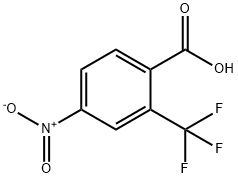
320-37-6
153 suppliers
$37.00/1G

694499-26-8
231 suppliers
$9.00/250mg
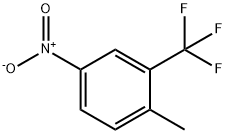
89976-12-5
205 suppliers
$8.00/1g

694499-26-8
231 suppliers
$9.00/250mg
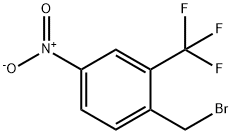
694499-22-4
69 suppliers
$50.00/50mg

694499-26-8
231 suppliers
$9.00/250mg