
7,8-dihydro-1H-furo[2,3-g]indole synthesis
- Product Name:7,8-dihydro-1H-furo[2,3-g]indole
- CAS Number:170728-95-7
- Molecular formula:C10H9NO
- Molecular Weight:159.18
![1H-Furo[2,3-g]indole-2,3-dione, 7,8-dihydro-](/CAS/20210111/GIF/327183-31-3.gif)
327183-31-3
2 suppliers
inquiry
![7,8-dihydro-1H-furo[2,3-g]indole](/CAS/20180906/GIF/170728-95-7.gif)
170728-95-7
1 suppliers
inquiry
Yield:170728-95-7 42.8%
Reaction Conditions:
Stage #1: 2,3,7,8-tetrahydro-1H-furo[2,3-g]indole-2,3-dionewith sodium tetrahydroborate in tetrahydrofuran at -20; for 0.333333 h;
Stage #2: with boron trifluoride diethyl etherate in tetrahydrofuran at -20 - 0; for 2.5 h;
Steps:
3 7,8-Dihydro-1H-furo[2,3-g]indole
A solution of 2,3,7,8-tetrahydro-1H-furo[2,3-g]indole-2,3-dione (9.05 g, 47.9 mmol) in tetrahydrofuran (100 mL) was stirred at -20° C. under an argon atmosphere. Solid sodium borohydride was added portionwise and the reaction was stirred for 20 min at -20° C. Boron trifluoride etherate (11.9 mL, 96 mmol) was added dropwise over 90 min, then the mixture was warmed to 0° C. and stirred for 1 h. The reaction was quenched with water (100 mL) and the solution was extracted with ethyl acetate (3*150 mL). The organic extracts were combined, dried (magnesium sulphate) and concentrated in vacuo to afford a yellow solid which was purified by column chromatography [SiO2; heptane-ethyl acetate (5:1)] to afford the title compound (3.2 g, 42.8%); analytical data as described above. The synthesis of Example 3 was completed as described above.
References:
US2005/187282,2005,A1 Location in patent:Page/Page column 10
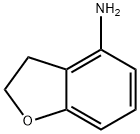
61090-37-7
90 suppliers
$40.00/50mg
![7,8-dihydro-1H-furo[2,3-g]indole](/CAS/20180906/GIF/170728-95-7.gif)
170728-95-7
1 suppliers
inquiry