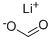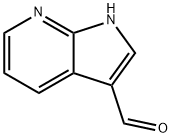
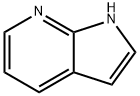
7-AZAINDOLE-3-CARBOXALDEHYDE synthesis
- Product Name:7-AZAINDOLE-3-CARBOXALDEHYDE
- CAS Number:4649-09-6
- Molecular formula:C8H6N2O
- Molecular Weight:146.15
271-63-6

64-19-7

4649-09-6
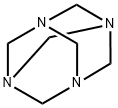
1H-pyrrolo[2,3-b]pyridine (11.80 g, 100 mmol) was suspended in 33% aqueous acetic acid (200 mL) at room temperature, followed by the addition of hexamethylenetetramine (16.8 g, 120 mmol). The reaction mixture was heated to 110-120 °C (oil bath temperature) and stirred continuously at this temperature for 15 hours. Upon completion of the reaction, the mixture was cooled to room temperature, at which point a large amount of solid precipitated. The suspension was poured into a 2 L beaker containing ice and the reaction flask was rinsed with 50 mL of water. Subsequently, saturated sodium bicarbonate solution was slowly added for neutralization. After completion of neutralization, the solid product was collected by filtration and washed with distilled water. The product was dried in air to give 1H-pyrrolo[2,3-b]pyridine-3-carboxaldehyde (9.5 g, 65% yield) as a white solid.EI-HRMS analysis results: m/e calculated value C8H6N2O (M+) 146.0480, measured value 146.0478.
Yield:4649-09-6 99.1%
Reaction Conditions:
with zinc diacetate in trifluoroacetic acid at 120; for 6 h;Solvent;Concentration;
Steps:
3 Example 3
The 10mmol 7-azaindole dissolved in 1L trifluoroacetic acid placed in a reaction vessel, was added 25mmol catalyst Stir to maintain the temperature at 120 °C, was added thereto 30mmol formic acid lithium salt, the reaction was refluxed 6h, The reaction was completed and purified to give 7-azaindole-3-carbaldehyde. Among them, the yield of 7-azaindole-3-carbaldehyde was 99.1%
References:
CN106279154,2017,A Location in patent:Paragraph 0021; 0023; 0024; 0025; 0027; 0029; 0031