
Sodium bicarbonate synthesis
- Product Name:Sodium bicarbonate
- CAS Number:144-55-8
- Molecular formula:CHNaO3
- Molecular Weight:84.01
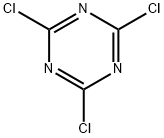
108-77-0
407 suppliers
$13.00/25g
![Carbamic acid, [[1-(2-amino-2-oxoethyl)cyclohexyl]methyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/20210305/GIF/227626-61-1.gif)
227626-61-1
0 suppliers
inquiry
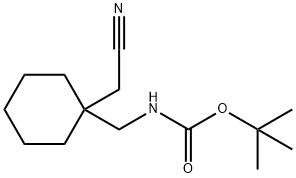
227626-62-2
4 suppliers
inquiry

144-55-8
1411 suppliers
$10.00/25g
Yield:227626-62-2 80%
Reaction Conditions:
in ice-water;N,N-dimethyl-formamide;
Steps:
4 (1-Cyanomethyl-cyclohexylmethyl)-carbamic acid tert-butyl ester (4)
(1-Cyanomethyl-cyclohexylmethyl)-carbamic acid tert-butyl ester (4) Cyanuric chloride (39.5 g, 0.214 mol) was added to (1-carbamoylmethyl-cyclohexylmethyl)-carbamic acid tert-butyl ester (3) (116 g, 0.429 mol) in 400 mL DMF. An ice-water bath was used to moderate the exotherm, and the reaction mixture was stirred at room temperature for 1.5 hours. The mixture was poured into ice-water containing 120 g (1.43 mol) NaHCO3 and was extracted 4*EtOAc. The extracts were washed 1*water, 2* brine and dried over Na2SO4. Evaporation yielded an oil which was taken up in 3:1 hexane/EtOAc and filtered through silica gel. Evaporation yielded white crystals (86.5 g, 80%); mp 54-58° C. 1H-NMR (CDCl3) δ1.3-1.5 (m, 19H), 2.30 (s, 2H), 3.15 (d, 2h, J=7.00 Hz), 4.60 (broad, 1H). MS (APCI) m/z 253 (M+1).
References:
US6518289,2003,B1

124-38-9
134 suppliers
$214.00/14L
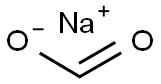
141-53-7
767 suppliers
$6.00/100g

144-55-8
1411 suppliers
$10.00/25g
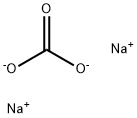
497-19-8
1488 suppliers
$14.00/1KG

144-55-8
1411 suppliers
$10.00/25g

124-38-9
134 suppliers
$214.00/14L

144-55-8
1411 suppliers
$10.00/25g

124-38-9
134 suppliers
$214.00/14L

7732-18-5
507 suppliers
$13.50/100ML
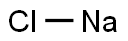
7647-14-5
1243 suppliers
$5.00/500g

144-55-8
1411 suppliers
$10.00/25g