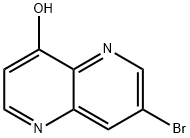
7-bromo-1,5-naphthyridin-4-ol synthesis
- Product Name:7-bromo-1,5-naphthyridin-4-ol
- CAS Number:97267-60-2
- Molecular formula:C8H5BrN2O
- Molecular Weight:225.04
![1,3-Dioxane-4,6-dione, 5-[[(5-bromo-3-pyridinyl)amino]methylene]-2,2-dimethyl-](/CAS/20210305/GIF/1151802-13-9.gif)
1151802-13-9
0 suppliers
inquiry

97267-60-2
10 suppliers
$200.00/0.25G
Yield:97267-60-2 70%
Reaction Conditions:
with diphenyl ether-biphenyl eutectic at 240; for 1.08333 h;
Steps:
3 3B . 7 -Bromo- 1 , 5 -naphthyridin-4-ol
To a DowThermA (100 mL), heated at 240 °C, was added in small portions over 5 min. 5-(((5-bromopyridin-3-yl)amino)methylene)-2,2-dimethyl-l,3-dioxane-4,6-dione (10 g, 30.6 mmol). With each portion, vigorous bubbling was observed (presumably as decarboxylation occurred). Halfway through the addition, the product began to precipitate. After the addition was complete, heating was continued for 5 min. and then for an additional lh as it cooled to 45°C. The precipitate was collected by vacuum filtration, rinsed with hexanes, and air-dried to afford a brown powder. This material was suspended in 150 mL of a 9: 1 mixture of EtOH and water and brought to reflux. The mixture was filtered hot and rinsed with EtOH. The solid was dried to afford 7-bromo- l,5-naphthyridin-4-ol (4.8 g, 70% yield) as a brown solid. LC/MS [M+H]+ = 224.8.
References:
WO2020/150114,2020,A1 Location in patent:Page/Page column 63-64
![5-[(5-METHOXY-PYRIDIN-3-YLAMINO)-METHYLENE]-2,2-DIMETHYL-[1,3]DIOXANE-4,6-DIONE](/CAS/20200401/GIF/847436-88-8.gif)
847436-88-8
6 suppliers
inquiry

97267-60-2
10 suppliers
$200.00/0.25G

97267-59-9
3 suppliers
inquiry

97267-60-2
10 suppliers
$200.00/0.25G
13535-01-8
390 suppliers
$3.00/1g

97267-60-2
10 suppliers
$200.00/0.25G
66093-18-3
8 suppliers
inquiry

97267-60-2
10 suppliers
$200.00/0.25G