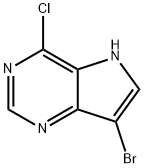
7-bromo-4-chloro-5H-pyrrolo[3,2-d]pyrimidine synthesis
- Product Name:7-bromo-4-chloro-5H-pyrrolo[3,2-d]pyrimidine
- CAS Number:1032650-41-1
- Molecular formula:C6H3BrClN3
- Molecular Weight:232.47
![7-BROMO-1,5-DIHYDRO-4H-PYRROLO[3,2-D]PYRIMIDIN-4-ONE](/CAS/GIF/93587-23-6.gif)
93587-23-6
![7-bromo-4-chloro-5H-pyrrolo[3,2-d]pyrimidine](/CAS2/GIF/1032650-41-1.gif)
1032650-41-1
Under argon protection, 7-bromo-1,5-dihydro-4H-pyrrolo[3,2-d]pyrimidin-4-one (100 mg, 0.5 mmol) was mixed with 6 mL of phosphorus oxytrichloride (POCl3) and the reaction was heated for 3 hours at 115 °C. After the reaction was completed, the mixture was cooled to room temperature and slowly poured into 300 mL of ice water with thorough stirring. The pH was adjusted to alkaline with potassium carbonate solution and subsequently extracted with ethyl acetate (EtOAc). The organic phases were combined, dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated under reduced pressure to afford 101 mg (93% yield) of the target product, 7-bromo-4-chloro-5H-pyrrolo[3,2-d]pyrimidine, as a beige solid, which could be used in the subsequent reaction without further purification.
![7-BROMO-1,5-DIHYDRO-4H-PYRROLO[3,2-D]PYRIMIDIN-4-ONE](/CAS/GIF/93587-23-6.gif)
93587-23-6
53 suppliers
$65.00/50mg
![7-bromo-4-chloro-5H-pyrrolo[3,2-d]pyrimidine](/CAS2/GIF/1032650-41-1.gif)
1032650-41-1
78 suppliers
$18.00/250mg
Yield: 93%
Reaction Conditions:
Stage #1:7-bromo-3,5-dihydropyrrolo[3,2-d]pyrimidin-4-one with trichlorophosphate at 115; for 3 h;
Stage #2: with potassium carbonate in water at 0 - 20;
Steps:
49
A mixture of 7-bromo-3,5-dihydropyrrolo[3,2-?]pyrimidin-4-one (100 mg, 0.5 mmol) and 6 mL of POCI3 under argon was warmed at 115°C. After 3 hours, the mixture was cooled to room temperature and poured over 300 mL of ice, stirred, made basic with potassium carbonate and extracted with EtOAc. The combined organic layers were dried, filtered, and concentrated in vacuo to provide 101 mg (93%) of the desired 7-bromo-4-chloro-5H-pyrrolo[3,2- djpyrimidine as beige solid which was used without further purification.
References:
Location in patent:Page/Page column 153
![4-CHLORO-5H-PYRROLO[3,2-D] PYRIMIDINE](/CAS/GIF/84905-80-6.gif)
84905-80-6
205 suppliers
$9.00/250mg
![7-bromo-4-chloro-5H-pyrrolo[3,2-d]pyrimidine](/CAS2/GIF/1032650-41-1.gif)
1032650-41-1
78 suppliers
$18.00/250mg
![1,5-DIHYDRO-4H-PYRROLO[3,2-D]PYRIMIDIN-4-ONE](/CAS/GIF/5655-01-6.gif)
5655-01-6
115 suppliers
$45.00/50mg
![7-bromo-4-chloro-5H-pyrrolo[3,2-d]pyrimidine](/CAS2/GIF/1032650-41-1.gif)
1032650-41-1
78 suppliers
$18.00/250mg
![ethyl 4-hydroxy-5H-pyrrolo[3,2-d]pyriMidine-7-carboxylate](/CAS/20150408/GIF/779326-74-8.gif)
779326-74-8
7 suppliers
inquiry
![7-bromo-4-chloro-5H-pyrrolo[3,2-d]pyrimidine](/CAS2/GIF/1032650-41-1.gif)
1032650-41-1
78 suppliers
$18.00/250mg
![1,5-DIHYDRO-4H-PYRROLO[3,2-D]PYRIMIDIN-4-ONE](/CAS/GIF/5655-01-6.gif)
5655-01-6
115 suppliers
$45.00/50mg
![7-bromo-4-chloro-5H-pyrrolo[3,2-d]pyrimidine](/CAS2/GIF/1032650-41-1.gif)
1032650-41-1
78 suppliers
$18.00/250mg