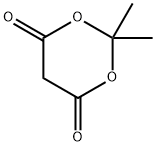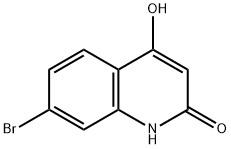
7-bromo-4-hydroxy-2(1H)-Quinolinone synthesis
- Product Name:7-bromo-4-hydroxy-2(1H)-Quinolinone
- CAS Number:100748-65-0
- Molecular formula:C9H6BrNO2
- Molecular Weight:240.05

1241675-38-6
5 suppliers
inquiry

100748-65-0
8 suppliers
inquiry
Yield:100748-65-0 90.9%
Reaction Conditions:
with potassium hexamethylsilazane in tetrahydrofuran at -78 - 10; for 2 h;Inert atmosphere;
Steps:
33.2 7-bromo-4-hydroxyquinolin-2(lH)-one
Two reactions of the same scale were performed in parallel. To solutions of potassium bis(trimethylsilyl)amide in THF (548 mL, 548 mmol) were added suspensions of methyl 2-acetamido-4-bromobenzoate (50 g, 183 mmol) in THF (500 mL) dropwise at -78°C under nitrogen atmosphere. After stirring at this temperature for lh the mixtures were allowed to warm to 10 °C within lh. The reactions were then combined and the mixture was quenched with water (3000 mL). The aqueous layer was washed with EtOAc (1000 mL x 2). The separated aqueous layer was cooled to 10°C and the pH was adjusted to 2.5-3.5 with the addition of 5N HCl aqueous solution resulting in a yellow precipitate. The solid was collected by filtration, washed with EtOAc (1000 mL) and dried under vacuum to give 7-bromo-4-hydroxyquinolin-2(lH)-one (80.0 g, 90.9% yield) as an off-white solid. LCMS: (FA) ES+ 241.7; NMR (400MHz, DMSO-dg) δ 11.52 (br s, 1H), 11.26 (s, 1H), 7.67 - 7.63 (m, 1H), 7.40 (s, 1H), 7.27 - 7.24 (m, 1H), 5.72 (s, 1H).
References:
WO2016/118565,2016,A1 Location in patent:Paragraph 00362
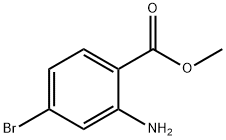
135484-83-2
183 suppliers
$6.00/1g

100748-65-0
8 suppliers
inquiry