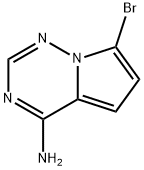
7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine synthesis
- Product Name:7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine
- CAS Number:937046-98-5
- Molecular formula:C6H5BrN4
- Molecular Weight:213.03
![PYRROLO[1,2-F][1,2,4]TRIAZIN-4-AMINE](/CAS/GIF/159326-68-8.gif)
159326-68-8
![7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine](/CAS/GIF/937046-98-5.gif)
937046-98-5
The general procedure for the synthesis of 4-amino-7-bromopyrrolo[2,1-f][1,2,4]triazines from 4-amino pyrrolo[2,1-f][1,2,4]triazines is as follows: a solution of anhydrous DMF (200 mL) containing 4-amino pyrrolo[2,1-f][1,2,4]triazines (21.0 g, 0.157 mol) was stirred and cooled to -20 °C, followed by the batch addition of 1,3-dibromo-5,5-dimethylglycolide (22.4 g, 0.078 mol), the addition process lasted 45 min. After completion of addition, stirring of the reaction mixture was continued for 45 min and the progress of the reaction was monitored by TLC (silica gel plate, GHLF, 5% CH3OH/CH2Cl2). Upon completion of the reaction, the reaction was quenched by the addition of saturated Na2SO3 solution (300 mL), the resulting suspension was stirred, and the solid product was collected by filtration. The filter cake was washed with water and dried by diafiltration. Subsequently, the dried solid was partitioned between ethyl acetate (1 L) and 5% sodium carbonate solution (1 L) to separate the organic and aqueous layers. The organic layer was washed with fresh 5% sodium carbonate solution and dried with magnesium sulfate. All organic phases were combined and filtered through a Magnesol pad and the filtrate was concentrated under vacuum to give the crude product 4-amino-7-bromopyrrolo[2,1-f][1,2,4]triazine (29.9 g, 90% yield). 21.5 g of the crude product was recrystallized in hot ethyl acetate (300 mL, 70 °C) to give a colorless solid (12.3 g) with about 2% of dibrominated byproducts. The structure of the product was confirmed by 1H-NMR (CD3OD) and mass spectrometry (LC/MS, +ESI): 1H-NMR δ 7.84 (s, 1H), 6.95 (d, 1H, J = 4.7 Hz), 6.71 (d, 1H, J = 4.7 Hz), 4.89 (s, 3H, -NH2 + H2O); MS m/z = 213.1 [M+H].
![PYRROLO[1,2-F][1,2,4]TRIAZIN-4-AMINE](/CAS/GIF/159326-68-8.gif)
159326-68-8
329 suppliers
$10.00/250mg
![7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine](/CAS/GIF/937046-98-5.gif)
937046-98-5
179 suppliers
$11.00/250mg
Yield:937046-98-5 91%
Reaction Conditions:
with 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione at -15 - -10; for 0.75 h;Large scale;
Steps:
5 The specific preparation method is:
Add 1000g of intermediate IV (7.45mol, 1.0eq) to a 20L three-necked bottle, add 10L of solvent, stir evenly, and lower the temperature to -15 ° C to obtain the reaction liquid for use;Dissolve 1065.7g of bromination reagent in 2L of solvent and slowly drop it into the reaction solution.Keep the temperature in the three-necked flask below -10 ° C and react for 45 minutes. After the reaction was monitored by TLC, 15 L of a 25% sodium sulfite solution was added to precipitate a solid.Filter with suction, wash the filter cake with water, dissolve and separate with a molar ratio of 1: 1 ethyl acetate and 5% sodium carbonate solution, and use a sodium carbonate solution for the organic phase.After washing with brine, drying, separation and concentration to obtain 1450 g of white solid, which is the final product (7-bromopyrrolo [2,1-f] [1,2,4] thiazin-4-amine).The purity of the final product is 97%, the yield is 91%, which contains 1.2% of the dibromide by-product. Among them, the reaction progress was monitored using TLC, and the developing agent used in the monitoring reaction was a mixed liquid of polyethylene and ethyl acrylate, and the molar ratio of polyethylene to ethyl acrylate was 3: 1.The bromination reagent added is one of N-bromosuccinimide, dibromohydantoin and NBS. In this embodiment, dibromohydantoin is preferably used. Other bromination reagents can also be selected without affecting the reaction product.The added solvent is one of anhydrous DMF, dichloromethane, and tetrahydrofuran; in this embodiment, anhydrous DMF is preferably used, and other bromination reagents can also be selected without affecting the reaction product.The molar ratio of intermediate IV to bromination reagent is 1.0: 0.5-1.5.
References:
Chengdu Aofei Biochemical Pin Co., Ltd.;Zhang Zhuo;Shen Mao;Zhao Linji CN110845502, 2020, A Location in patent:Paragraph 0058-0064
![7-Iodopyrrolo[2,1-f][1,2,4]triazin-4-amine](/CAS/20200611/GIF/1770840-43-1.gif)
1770840-43-1
277 suppliers
inquiry
![7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine](/CAS/GIF/937046-98-5.gif)
937046-98-5
179 suppliers
$11.00/250mg
937046-96-3
107 suppliers
inquiry
![7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine](/CAS/GIF/937046-98-5.gif)
937046-98-5
179 suppliers
$11.00/250mg