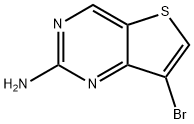
7-Bromothieno[3,2-d]pyrimidin-2-amine synthesis
- Product Name:7-Bromothieno[3,2-d]pyrimidin-2-amine
- CAS Number:1293987-58-2
- Molecular formula:C6H4BrN3S
- Molecular Weight:230.09
![7-Bromo-2-chlorothieno[3,2-d]pyrimidine](/CAS2/GIF/1152475-42-7.gif)
1152475-42-7
84 suppliers
$82.00/100mg
![7-Bromothieno[3,2-d]pyrimidin-2-amine](/CAS/20210111/GIF/1293987-58-2.gif)
1293987-58-2
7 suppliers
inquiry
Yield:1293987-58-2 60%
Reaction Conditions:
with ammonia in isopropyl alcohol at 100; for 48 h;Sealed reactor;
Steps:
19.1
7-Bromo-2-chlorothieno[3,2-d]pyrimidine (1.0 g, 4.03 mmol) was dissolved in 2.0 M ammonia isopropanol (10 mL) and stirred at 100 °C for 48 hours in a sealed reactor. The reaction mixture was cooled to room temperature and then concentrated. Purification by chromatography (50% ethyl acetate/hexane) yielded a brown solid compound (560 mg, 60% yield). [0253] 1H NMR (400 MHz, DMSO-d 6) δ 8.95 (s, 1H), 8.36 (s, 1H), 6.89 (s, 2H), MS m/z: 230.26, 232.26 [M+1].
References:
WO2011/49332,2011,A2 Location in patent:Page/Page column 29
![1,3-Dihydrothiopheno[3,2-d]pyrimidine-2,4-dione](/CAS/GIF/16233-51-5.gif)
16233-51-5
163 suppliers
$6.00/250mg
![7-Bromothieno[3,2-d]pyrimidin-2-amine](/CAS/20210111/GIF/1293987-58-2.gif)
1293987-58-2
7 suppliers
inquiry
22288-78-4
391 suppliers
$11.00/5g
![7-Bromothieno[3,2-d]pyrimidin-2-amine](/CAS/20210111/GIF/1293987-58-2.gif)
1293987-58-2
7 suppliers
inquiry
![7-bromothieno[3,2-d]pyrimidine-2,4(1H,3H)-dione](/CAS/20180808/GIF/41102-02-7.gif)
41102-02-7
23 suppliers
inquiry
![7-Bromothieno[3,2-d]pyrimidin-2-amine](/CAS/20210111/GIF/1293987-58-2.gif)
1293987-58-2
7 suppliers
inquiry
![7-Bromo-2,4-dichloro-thieno[3,2-d]pyrimidine](/CAS2/GIF/41102-25-4.gif)
41102-25-4
54 suppliers
$130.00/250mg
![7-Bromothieno[3,2-d]pyrimidin-2-amine](/CAS/20210111/GIF/1293987-58-2.gif)
1293987-58-2
7 suppliers
inquiry