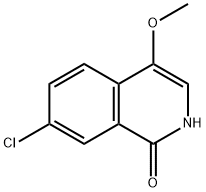
1(2H)-Isoquinolinone, 7-chloro-4-methoxy- synthesis
- Product Name:1(2H)-Isoquinolinone, 7-chloro-4-methoxy-
- CAS Number:630423-35-7
- Molecular formula:C10H8ClNO2
- Molecular Weight:209.63

67-56-1
789 suppliers
$9.00/25ml
1210474-91-1
0 suppliers
inquiry

630423-35-7
32 suppliers
$175.00/250mg
Yield:630423-35-7 89.95%
Reaction Conditions:
with methanesulfonic acid at 3 - 60;
Steps:
8
To a 12 L RBF (fitted with glycol-cooled condenser, gas inlet adaptor, stir bar and heating mantle) was added the starting alcohol (483.6 g) and Methanol (2.68 kg).The slurry of brown solids was cooled to 3° C. in an ice/water bath.Methanesulfonic Acid (2.38 kg) was added via addition funnel over about 25 minutes (VERY EXOTHERMIC). During the addition, the solids appeared to partially dissolve and the reaction mixture turned reddish brown.The reaction mixture was heated to 60° C.Heating was continued at 60° C. and the reaction monitored by HPLC.HPLC results:; After 47 hours, the reaction mixture was cooled to room temperature.The mixture was filtered through diatomaceous earth (Celite) to remove solids since the reverse quench was going to be done via addition funnel and residual solid could block the stopcock.Ammonium Hydroxide (3.09 kg) was cooled using ice/water in a 22 L flask to 1.6° C.The reaction mixture was added via dropping funnel to the ammonium hydroxide, keeping the temperature <30° C.; The reaction flask was rinsed with 250 mL methanol to remove the remaining reaction mixture and the rinse transferred to the quench vessel.The resulting slurry was held at room temperature. Quantitative samples of the supernatant taken after 1 hour, 3 hours, and 5 hours all showed the same level of product remaining in the mother liquors and the same impurity profile. In future, samples will be taken after cooling to room temperature and after 2 hours to determine whether or not the crystallization is complete.The slurry was filtered through No.54 Whatman paper, 24 cm diameter on a Buchner funnel.The cake was washed with 5 L of water; each wash took about 10 minutes.Cake dimensions before compression were 25 cm diameter, 5.5 cm thick for a volume of 2700 mL.The solid was very slow to deliquor since the cake tends to crack very easily. The crystals are very fine and form a cake which does not release solvents easily.The solid was dried under vacuum at 40° C. This took several days.The desired product (466.2 g; 89.95% yield) was isolated. LOD of the solid was 0.46%. HPLC AP was 97.33 with 2.1 P starting material and 2 other unknown impurities.
References:
US2010/56792,2010,A1 Location in patent:Page/Page column 11-12

67-56-1
789 suppliers
$9.00/25ml
24188-74-7
102 suppliers
$43.00/100mg

630423-35-7
32 suppliers
$175.00/250mg