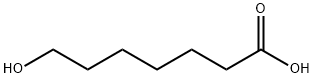
7-HYDROXYHEPTANOIC ACID synthesis
- Product Name:7-HYDROXYHEPTANOIC ACID
- CAS Number:3710-42-7
- Molecular formula:C7H14O3
- Molecular Weight:146.18

13175-44-5

3710-42-7
The general procedure for the synthesis of 7-hydroxyheptanoic acid from 7-octen-1-ol was as follows: a solution of 7-octen-1-ol (1 mL, 0.88 g, 6.66 mmol, 1 eq.) in MeCN (15.8 mL, 18 mL/g) and H2O (1.8 mL, 2 mL/g) was added to a reaction vial. The reaction mixture was cooled to 0 °C and ozone was passed at a rate of 12 h at room temperature. The reaction was carried out under the protection of nitrogen at 1 atm. Subsequently, a NaHSO3 solution was prepared by dissolving Na2S2O5 (2.77 g, 14.58 mmol, 2.19 eq.) in H2O (14 mL) and this solution was slowly added to the reaction mixture, keeping the internal temperature below 35°C. After the addition was completed, the mixture was stirred for 10 minutes. EtOAc (10 mL) was added to the reaction mixture and the organic and aqueous layers were separated. The aqueous layer was further extracted with EtOAc, and all the organic layers were combined, dried with MgSO4, filtered and concentrated to give the colorless oily product 7-hydroxyheptanoic acid (0.95 g, 95% yield).

629-30-1
273 suppliers
$8.00/1g

3710-42-7
87 suppliers
$24.00/100mg
Yield:3710-42-7 76%
Reaction Conditions:
with dihydrogen peroxide;sodium hydroxide in water at 79.84; for 8 h;Schlenk technique;
Steps:
2.3. Catalytic testing
General procedure: All experiments to test the catalytic activity were performed in a Schlenk tube (50mL vol.) attached to a condenser. The catalytic activity was evaluated for HDO oxidation in basic aqueous media with H2O2 as oxidant to obtain HCA. In a typical reaction procedure, aliphatic diol (0.5mmol) and catalyst (25mg) were weighed and dispersed in deionized water (3.5mL) in a Schenk tube. 30% H2O2 (0.75mL) and 0.5M NaOH (0.75mL) were added to the above mixture, and then the Schlenk tube was mounted on a preheated oil bath at 353K. The mixture was allowed to react for various time intervals with continuous magnetic stirring (500 rpm). After the reaction, a part of the resultant solution was diluted 20 times with an aqueous H2SO4 (10mM) solution, and the catalyst was filtered off using a 0.20μm filter (Milex-LG). The obtained filtrate was analyzed by high performance liquid chromatography (HPLC, WATERS 600) using an Aminex HPX-87H column (Bio-Rad Laboratories, Inc.) attached to a refractive index detector. An aqueous 10mM H2SO4 solution (eluent) was run through the column (maintained at 323K) at a flow rate of 0.5mLmin-1. The conversion and yield(s) were determined with a calibration curve method using commercial products.
References:
Tuteja, Jaya;Nishimura, Shun;Ebitani, Kohki [Catalysis Today,2016,vol. 265,p. 231 - 239]

13175-44-5
83 suppliers
$45.00/50mg

3710-42-7
87 suppliers
$24.00/100mg
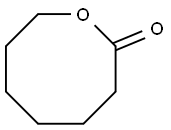
539-87-7
16 suppliers
inquiry

3710-42-7
87 suppliers
$24.00/100mg

4286-55-9
307 suppliers
$15.00/10g

124-38-9
134 suppliers
$175.00/23402

3710-42-7
87 suppliers
$24.00/100mg
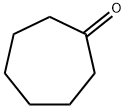
502-42-1
218 suppliers
$10.00/1g

3710-42-7
87 suppliers
$24.00/100mg