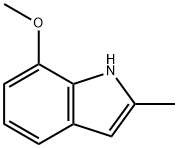
7-methoxy-2-methyl-1H-indole synthesis
- Product Name:7-methoxy-2-methyl-1H-indole
- CAS Number:53512-46-2
- Molecular formula:C10H11NO
- Molecular Weight:161.2
111258-23-2
150 suppliers
$21.00/100mg

53512-46-2
32 suppliers
inquiry
Yield:53512-46-2 99%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran;1,4-dioxane at 0; for 16 h;Reflux;
Steps:
2-(l-(4-chIorobenzyI)-4-methoxy-2-methyl-lH-indol-3-yl)-N-(2-methoxypyridin-4-yl)- 2-oxoacetamide (45); [00314] To a 0 °C solution of methyl 4-methoxy- 1 H-indole-2-carboxylate ( 1.00 g, 4.87 mmol) in 1,4-dioxane (20 mL) was added, dropwise, a 2M in tetrahydrofuran solution of lithium aluminum hydride (12.2 mL, 24.4 mmol). The reaction mixture was warmed to room temperature for an hour then heated to reflux for 15 hours after which the reaction was complete. The reaction mixture was then cooled to 0 °C, after which ice water (5 mL) was cautiously added, followed by an additional 20 mL of water. The reaction mixture was extracted with ethyl acetate (3 x 20 mL), washed by saturated sodium chloride solution (3 x 20 mL), dried (sodium sulfate), filtered and concentrated to afford 4-methoxy-2-methyl-lH- indole (0.780 g, 4.84 mmol, 99 % yield) as a solid.[00315] 2-(l-(4-chlorobenzyl)-4-methoxy-2-methyl-lH-indol-3-yl)-N-(2- methoxypyridin-4-yl)-2-oxoacetamide was synthesized as a yellow solid starting from 4- methoxy-2-methyl-lH-indole using general procedure G. 1H NMR (400 MHz, CDC13) δ (ppm): 8.45 (br. s, IH), 8.13 (d, IH), 7.28 (d, 2H), 7.10-7.21 (m, 3H), 6.97 (d, 2H), 6.85 (d, IH), 6.62 (d, IH), 5.35 (s, 2H), 3.95 (s, 3H), 3.71 (s, 3H), 2.56 (s, 3H).
References:
WO2012/88469,2012,A1 Location in patent:Page/Page column 131
24610-33-1
45 suppliers
$113.00/100mg

53512-46-2
32 suppliers
inquiry
91-23-6
19 suppliers
$10.00/1g
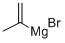
13291-18-4
173 suppliers
$133.00/25ml

53512-46-2
32 suppliers
inquiry
![1H-Indole, 7-methoxy-2-methyl-1-[(4-methylphenyl)sulfonyl]-](/CAS/20210305/GIF/1192805-50-7.gif)
1192805-50-7
0 suppliers
inquiry

53512-46-2
32 suppliers
inquiry
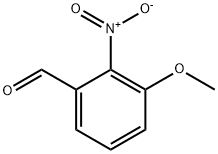
53055-05-3
121 suppliers
$60.00/100mg

53512-46-2
32 suppliers
inquiry