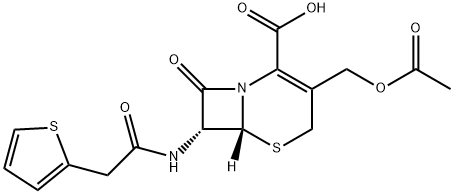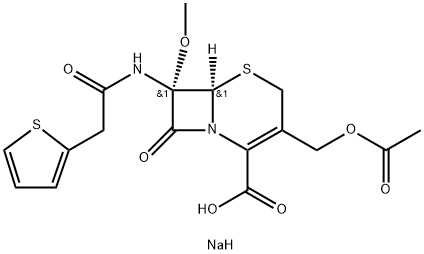
7α-Methoxycephalotin Monosodium Salt synthesis
- Product Name:7α-Methoxycephalotin Monosodium Salt
- CAS Number:35565-07-2
- Molecular formula:C17H19N2NaO7S2
- Molecular Weight:450.46
Yield:-
Reaction Conditions:
with tert-butylhypochlorite;methyl tri-n-octyl ammonium hydrogen sulfate in methanol;dichloromethane at -90 - -80;
Steps:
2
A 500 mL four-necked flask was charged with 150 mL of methanol, 10.8 g (0.20 mol) of sodium methoxide, stirred and dissolved, and cooled to -20 to -25 ° C. To a 2000 mL four-necked flask was charged 600 mL of dichloromethane, 39.6 g (0.10 mol) of cephalothin, 4.7 g (0.01 mol) of trioctylmethylammonium hydrogen sulfate, Stirring down to -80 ~ -90 , Drop the methanol solution with a good amount of sodium methoxide, Dropping process temperature control -80 ~ -90 , Drop finished, A further 13.5 g (0.12 mol) of t-butyl hypochlorite prepared in the first step was added dropwise, Dropping process temperature control -80 ~ -90 , Drop finished, Temperature control -80 ~ -90 stirring reaction 0.5 ~ 1h, To obtain a solution of a methoxy group, (HPLC detection of cefotaxime Reaction completed
References:
CN104402909,2017,B Location in patent:Paragraph 0030; 0031