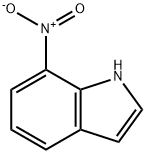
7-Nitroindole synthesis
- Product Name:7-Nitroindole
- CAS Number:6960-42-5
- Molecular formula:C8H6N2O2
- Molecular Weight:162.15
6960-45-8

6960-42-5
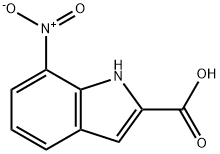
Example 4: Preparation of Compound 4 (7-nitro-1H-indole); Compound 3 (7-nitroindole-2-carboxylic acid, 1.63 g, 7.91 mmol) was dissolved in quinoline (13 mL) followed by addition of copper oxide (CuO, 0.19 g). The reaction mixture was stirred and heated to 194 °C. During heating, gas was observed to escape. After 2 hours of reaction, gas production stopped and the completion of the reaction was confirmed by thin layer chromatography (TLC). The reaction solution was slowly poured into a dilute hydrochloric acid solution prepared from concentrated hydrochloric acid (21.3 mL) and cold water (42.6 mL) and stirred to produce a black precipitate. The reaction mixture was filtered to separate the black precipitate and the filtrate was extracted with ether. The organic phases were combined and washed sequentially with saturated sodium bicarbonate (NaHCO3) solution and deionized water. The organic layer was dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated to give a yellow solid. The solid was recrystallized in ethanol (EtOH) to give an acicular light yellow solid, compound 4 (7-nitro-1H-indole, 0.87 g, 69% yield). Melting point 96-97 °C (literature value 95-96 °C); 1H NMR (200 MHz, CDCl3) δ 6.63 (dd, J = 2.2, 1.0 Hz, 1H, ArH), 7.08-7.18 (m, 1H, ArH), 7.32 (t, J = 3.0 Hz, 1H, ArH), 7.90 (d, J = 7.8 Hz, 1H, ArH), 8.08 (d, J = 8.1 Hz, 1H, ArH), 9.88 (s, 1H, NH); 13C NMR (50 MHz, acetone-d6) δ 103.7, 118.9, 119.2, 128.4, 129.1, 129.3, 132.9, 133.5; mass spectrometry (EI) m/z 162 (M+, 100%), 116 (M-46 ), 116 (M-46, 87%), 104 (M-58, 41%), 89 (M-73, 53%).
Yield:6960-42-5 90%
Reaction Conditions:
with C14H22B10Br2FeN2 in toluene at 20; for 4 h;
Steps:
6 The ferrous complex prepared in Example 1 was used as a catalyst to catalyze the synthesis of indole derivatives:
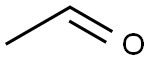
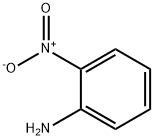
Toluene (toluene) solution of divalent ferrous complex (0.003mmol) was added to 2-nitroaniline (1mmol), then acetaldehyde (1mmol) was added, and the reaction was carried out at room temperature for 240 minutes. After completion, the concentrated reaction solution was directly passed through a silica gel column Chromatographic separation, drying to the same quality, the corresponding indole derivative C8H6N2O2 (yield 90%)
References:
CN113185444,2021,A Location in patent:Paragraph 0049-0052

6960-45-8
166 suppliers
$7.00/25mg

6960-42-5
244 suppliers
$10.00/1g
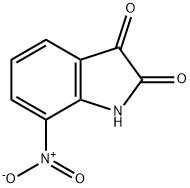
112656-95-8
93 suppliers
$6.00/250mg

6960-42-5
244 suppliers
$10.00/1g
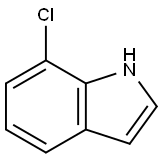
53924-05-3
309 suppliers
$14.14/250mgs:

6960-42-5
244 suppliers
$10.00/1g
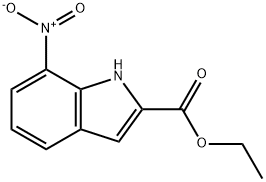
6960-46-9
111 suppliers
$7.00/100mg

6960-42-5
244 suppliers
$10.00/1g