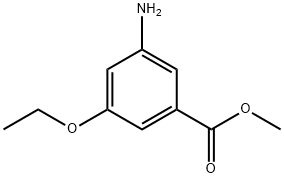
Benzoic acid, 3-amino-5-ethoxy-, methyl ester (9CI) synthesis
- Product Name:Benzoic acid, 3-amino-5-ethoxy-, methyl ester (9CI)
- CAS Number:706792-04-3
- Molecular formula:C10H13NO3
- Molecular Weight:195.22
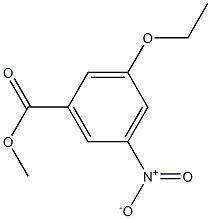
1375150-03-0

706792-04-3
General procedure for the synthesis of methyl 3-amino-5-ethoxybenzoate from methyl 3-ethoxy-5-nitrobenzoate: to methyl 3-ethoxy-5-nitrobenzoate (6.1 kg, 27.1 mol) prepared in step 2, methanol (54 L) and purified water (54 L) were added, followed by 10% palladium/carbon catalyst (6.1 kg, 10% wt/wt) . The reaction was carried out at room temperature with stirring in a hydrogen atmosphere at 4 atmospheres for 2.5 hours. Upon completion of the reaction, acetone (54 L) was added to dilute the reaction mixture, followed by removal of the 10% palladium/carbon catalyst by filtration. Methanol and acetone were removed by distillation under reduced pressure. The resulting solid was filtered and washed with pure water to give the final methyl 3-amino-5-ethoxybenzoate (4.88 kg, 92.2% yield, yellow solid). The product was characterized by 1H-NMR (400 MHz, CDCl3) with the following chemical shifts: δ 6.95 (s, 2H), 6.39 (s, 1H), 4.01 (q, J=6.8Hz, 2H), 3.87 (s, 3H), 3.76 (s, 2H), 1.38 (t, J=6.8Hz, 3H).

1375150-03-0
12 suppliers
inquiry

706792-04-3
40 suppliers
$9.00/250mg
Yield:706792-04-3 92.2%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol;water at 20; under 3040.2 Torr; for 2.5 h;Large scale;
Steps:
1.3 Step 3:
Preparation of methyl 3-amino-5-ethoxybenzoate
In the compound prepared in Step 2 (6.1 kg, 27.1 mol), methanol (54 L) and purified water (54 L) were added, and 10%-palladium/carbon (6.1 kg, 10% wt/wt) was added thereto, followed by stirring under hydrogen gas conditions at 4 atm for 2.5 hours at room temperature.
After securing the completion of the reaction, acetone (54 L) was added, and filtering was performed to remove 10%-palladium/carbon.
Methanol and acetone were distilled off under a reduced pressure.
The solid thus produced was filtered and washed with purified water to obtain the title compound (4.88 kg, yield: 92.2%, yellow solid).
1H-NMR (400 MHz, CDCl3) δ 6.95 (s, 2H), 6.39 (s, 1H), 4.01 (q, J=6.8 Hz, 2H), 3.87 (s, 3H), 3.76 (s, 2H), 1.38 (t, J=6.8 Hz, 3H)
References:
US2014/350007,2014,A1 Location in patent:Paragraph 0082; 0083; 0084
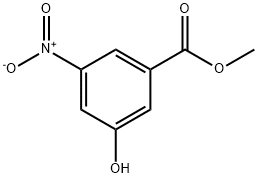
55076-32-9
54 suppliers
$125.00/250mg

706792-04-3
40 suppliers
$9.00/250mg
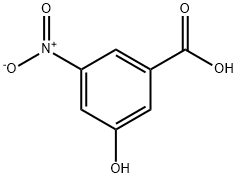
78238-14-9
106 suppliers
$21.00/1g

706792-04-3
40 suppliers
$9.00/250mg