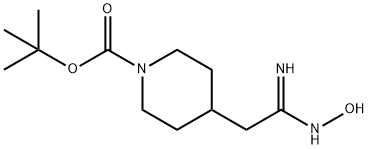
TERT-BUTYL 4-[(2Z)-2-AMINO-2-(HYDROXYIMINO)ETHYL]PIPERIDINE-1-CARBOXYLATE synthesis
- Product Name:TERT-BUTYL 4-[(2Z)-2-AMINO-2-(HYDROXYIMINO)ETHYL]PIPERIDINE-1-CARBOXYLATE
- CAS Number:713147-49-0
- Molecular formula:C12H23N3O3
- Molecular Weight:257.33
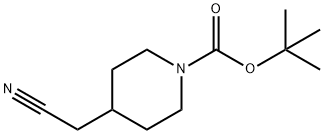
256411-39-9
93 suppliers
inquiry
![TERT-BUTYL 4-[(2Z)-2-AMINO-2-(HYDROXYIMINO)ETHYL]PIPERIDINE-1-CARBOXYLATE](/CAS/GIF/713147-49-0.gif)
713147-49-0
12 suppliers
inquiry
Yield:713147-49-0 81.36%
Reaction Conditions:
with hydroxylamine hydrochloride;potassium carbonate in ethanol;water at 20; for 48 h;
Steps:
16
4-Cyanomethyl-piperidine-1 -carboxylic acid ferf-butyl ester (compound of Example 15; 2.3 g, 10.2 mmol), anhydrous potassium carbonate (2.27 g, 16.4 mmol) and hydroxylamine hydrochloride (2.14 g, 30.8 mmol) in ethanol: water (25 : 4 ml.) was stirred at room temperature for 48 h. The reaction mixture was filtered, solvent evaporated and the residue obtained was purified by column chromatography (silica gel, 8 % ethyl acetate in petroleum ether) to afford the title compound.Yield: 2.14 g (81 .36 %); 1 H NMR (MeOD, 300 MHz): δ 4.04 (m, 2H), 2.73 (m, 2H), 1 .99 (d, 2H), 1 .77 (m, 1 H), 1 .67 (m, 2H), 1 .44 (s, 9H), 1 .10 (m, 2H); MS (ES+): 258 (M+1 ).
References:
WO2011/104680,2011,A1 Location in patent:Page/Page column 74

79099-07-3
0 suppliers
$5.00/5g
![TERT-BUTYL 4-[(2Z)-2-AMINO-2-(HYDROXYIMINO)ETHYL]PIPERIDINE-1-CARBOXYLATE](/CAS/GIF/713147-49-0.gif)
713147-49-0
12 suppliers
inquiry
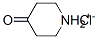
41979-39-9
0 suppliers
$15.00/10mg
![TERT-BUTYL 4-[(2Z)-2-AMINO-2-(HYDROXYIMINO)ETHYL]PIPERIDINE-1-CARBOXYLATE](/CAS/GIF/713147-49-0.gif)
713147-49-0
12 suppliers
inquiry
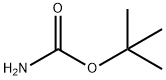
4248-19-5
379 suppliers
$5.00/5g
![TERT-BUTYL 4-[(2Z)-2-AMINO-2-(HYDROXYIMINO)ETHYL]PIPERIDINE-1-CARBOXYLATE](/CAS/GIF/713147-49-0.gif)
713147-49-0
12 suppliers
inquiry