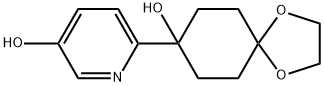
3-Hydroxy-6-(8-hydroxy-1,4-dioxaspiro[4.5]decan-8-yl)pyridine synthesis
- Product Name:3-Hydroxy-6-(8-hydroxy-1,4-dioxaspiro[4.5]decan-8-yl)pyridine
- CAS Number:713526-59-1
- Molecular formula:C13H17NO4
- Molecular Weight:251.28
55717-45-8
291 suppliers
$5.00/250mg
![1,4-Dioxaspiro[4.5]decan-8-one](/CAS/GIF/4746-97-8.gif)
4746-97-8
491 suppliers
$5.00/1g
![3-Hydroxy-6-(8-hydroxy-1,4-dioxaspiro[4.5]decan-8-yl)pyridine](/CAS/20180703/GIF/713526-59-1.gif)
713526-59-1
10 suppliers
inquiry
Yield:713526-59-1 79%
Reaction Conditions:
Stage #1: 6-bromo-pyridin-3-olwith n-butyllithium in tetrahydrofuran;hexane at -78; for 0.333333 h;
Stage #2: with sec.-butyllithium in tetrahydrofuran;hexane;cyclohexane at -78; for 1 h;
Stage #3: cyclohexanedione monoethylene ketal in tetrahydrofuran;hexane;cyclohexane at -78; for 1 h;
Steps:
3.3-A
To a solution of 6-bromopyridin-3-ol (8.0 g, 46 mmol) in THF (150 ML) was added dropwise a 1.4 M solution of n-butyllithium in hexane (33 ML, 46 mmol) at-78 °C and the mixture was stirred for 20min. A 0.90 M solution of sec-butyllitium in cyclohexane (77 ml, 69 mmol) was added dropwise and the mixture was stirred at- 78 °C for 1 hour. To the mixture was added a solution of 1, 4-dioxaspiro [4.5] decan- 8-one (11 g, 69 mmol) in THF (70 ml) dropwise at-78 °C and the mixture was stirred AT-78 °C FOR 1 hour. Sat. NAH2PO4 aq. (100 ml) was slowly added to the mixture and the mixture was warmed to room temperature. The organic layer was separated and the aqueous layer was extracted with ethyl acetate (100 ml x 3). The combined extracts were washed with brine, dried over MGS04, and evaporated in vacuum. The residue was purified by column chromatography on silica gel (hexane: ethyl acetate = 1: 2 as eluent) to afford the titled compound as a white powder. (9.1 g, 79%) 'H NMR (DMSO-d6) 8 : 9.67 (s, 1H), 8.02 (d, J = 2.9 Hz, 1H), 7.45 (d, J = 8.5 Hz, 1H), 7.12 (dd, J = 8.5, 2.9 Hz, 1H), 4.93 (s, 1H), 3.87 (s, 4H), 2.22-2. 04 (m, 2H), 1.97-1. 81 (m, 2H), 1.61-1. 44 (m, 4H) ppm.
References:
WO2004/54579,2004,A1 Location in patent:Page/Page column 55