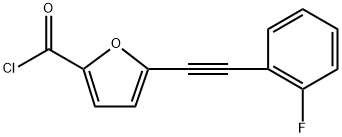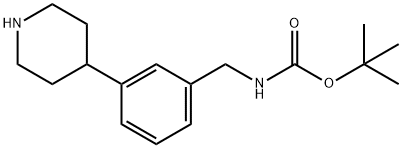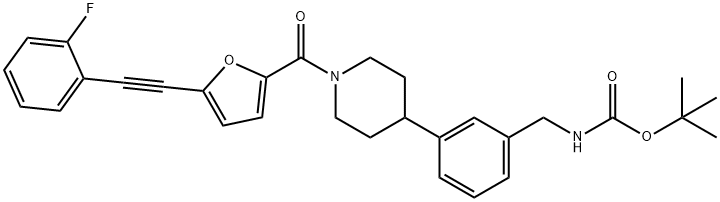
tert-butyl 3-(1-(5-((2-fluorophenyl)ethynyl)furan-2-carbonyl)piperidin-4-yl)benzylcarbaMate synthesis
- Product Name:tert-butyl 3-(1-(5-((2-fluorophenyl)ethynyl)furan-2-carbonyl)piperidin-4-yl)benzylcarbaMate
- CAS Number:725228-52-4
- Molecular formula:C30H31FN2O4
- Molecular Weight:502.58
Yield:725228-52-4 96%
Reaction Conditions:
with triethylamine in dichloromethane at 8 - 12; for 4 h;
Steps:
2.J J. (3-{1-[5-(2-Fluorophenylethynyl)-furan-2-carbonyl]-piperidin-4-yl}-benzyl)-carbamic acid tert-butyl ester
To a solution of (3-Piperidin-4-yl-benzyl)-carbamic acid tert-butyl ester (151.1 g, 0.52 mol) and triethylamine (93 mL, 0.66 mol) in methylene chloride (2 L), under N2, is added dropwise, over 1 hour, at 10°C (2°C), a solution of 5- (2-FLUOROPHENYLETHYNYL)-FURAN-2-CARBONYL chloride in methylene chloride (0.6 L). Following the end of addition the ice-bath is removed and the solution is stirred at room temperature for 3 hours. The solution is washed with water (0.9 L), 0.25 N AQ. HCI (0.9 L), saturated aq. NAHCO3 (0.5 L), brine (0.5 L), dried (MGS04), and concentrated in VACUO TO 291.6 G OF A FOAMING OIL. THE RESIDUE IS CRYSTALLIZED FROM METHYLENE CHLORIDE (0.5 L) /HEPTANE (1.25 L) mixture. The solid is isolated by filtration, rinsed with 0.9 L of a heptane/methylene chloride (2: 1) mixture, and dried at 35°C under 50 mmHg for 16 hours to give 162.6 g of the title compound as a white solid: mp 111-113°C ;'H NMR (300 MHz, CDC13) 6 7.53 (td, 1 H), 7.41-7. 28 (M, 2 H), 7.18-7. 08 (M, 5 H), 7.04 (d, 1 H, J = 3.6 Hz), 6.76 (d, 1 H, J= 3. 6 HZ), 4.83 (bs, I H), 4.72 (bm, 2 H), 4.31 (d, 2 H, J = 5.6 Hz), 3.1 (bs, 1 H), 2.82 (M, 2 H), 1.96 (M, 2 H), 1.79 (td, 2 H, J = 12.8, 4.0 Hz), 1.46 (s, 9 H); LC-MS (ESI) M/Z 503 (M+ + 1,100). Anal. Calcd for C30H3, N204F (502.59) : C, 71.70 ; H, 6.22 ; N, 5.57. Found: C, 71.66 ; H, 6.51 ; N, 5.52. The mother liquor is partially concentrated removing the bulk of methylene chloride. The resulting solid suspension is isolated by filtration, rinsed with heptane and dried (35°C, 50 mm Hg, 40 hours), to afford an additional 86.5 g of the title compound as an off-white solid: mp 107-110°C.'H NMR and LC-MS data as above. Anal. Found: C, 71.39 ; H, 6.48 ; N, 5.37. Combined yield for 2 crops: 249. 1 G, 96%.
References:
WO2004/60884,2004,A1 Location in patent:Page 29