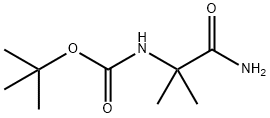
tert-Butyl (1-amino-2-methyl-1-oxopropan-2-yl)carbamate synthesis
- Product Name:tert-Butyl (1-amino-2-methyl-1-oxopropan-2-yl)carbamate
- CAS Number:73470-46-9
- Molecular formula:C9H18N2O3
- Molecular Weight:202.25
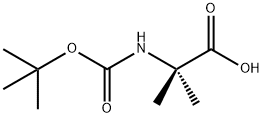
30992-29-1

73470-46-9
2-((tert-butoxycarbonyl)amino)-2-methylpropanoic acid (CAN: 30992-29-1, 20 g, 98 mmol) was used as a raw material and mixed with di-tert-butyl dicarbonate (CAN: 24424-99-5, 27.67 g, 147 mmol) in acetonitrile (500 mL) and pyridine (4.6 mL) was added as a catalyst. The reaction mixture was stirred at room temperature for 20 minutes. Subsequently, ammonia (10 mL) was slowly added dropwise over 20 minutes. After the dropwise addition, the reaction mixture was continued to be stirred for 4 hours. Upon completion of the reaction, most of the solvent was removed by distillation under reduced pressure. The precipitated solid was collected by filtration and washed with acetonitrile. Finally, the solid was dried under reduced pressure to afford the target product boc-2-aminoisobutyric acid amide (17.5 g, 88% yield) as a white solid. Mass spectrometry (EI) analysis showed: m/e 225.1 [M + Na]+.

30992-29-1
292 suppliers
$6.00/5g

73470-46-9
24 suppliers
$59.00/100mg
Yield:73470-46-9 88%
Reaction Conditions:
Stage #1: Boc-Aib-OHwith pyridine;di-tert-butyl dicarbonate in acetonitrile at 20; for 0.333333 h;
Stage #2: with ammonia in water;acetonitrile; for 4.33333 h;
Steps:
12.a
A mixture of 2-(tert-butoxycarbonylamino)-2-methylpropanoic acid (CAN: 30992-29-1, 20 g, 98 mmol), di-tert-butyl dicarbonate (CAN 24424-99-5, 27.67 g, 147 mmol) and pyridine (4.6 mL) in acetonitrile (500 mL) was stirred at room temperature for 20 min. Ammonia (10 mL) was added dropwise for 20 min. The resulting reaction mixture was stirred for 4 h. After removal of most of the solvent under reduced pressure, the solid was filtered off and washed with acetonitrile. The solid was brought to dryness under reduced pressure to give the title compound (17.5 g, 88%) as white solid; MS (EI): m/e 225.1 [M+Na]+.
References:
US2012/316147,2012,A1 Location in patent:Page/Page column 33-34

24424-99-5
869 suppliers
$13.50/25G

30992-29-1
292 suppliers
$6.00/5g

73470-46-9
24 suppliers
$59.00/100mg