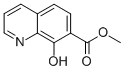
7-Quinolinecarboxylicacid,8-hydroxy-,methylester(6CI,9CI) synthesis
- Product Name:7-Quinolinecarboxylicacid,8-hydroxy-,methylester(6CI,9CI)
- CAS Number:73776-20-2
- Molecular formula:C11H9NO3
- Molecular Weight:203.196

67-56-1
790 suppliers
$9.00/25ml
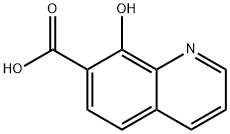
19829-79-9
86 suppliers
$45.00/50mg

73776-20-2
11 suppliers
inquiry
Yield:73776-20-2 100%
Reaction Conditions:
with sulfuric acid for 6 h;Reflux;
Steps:
2.2. Methyl 8-hydroxyquinoline-7-carboxylate (OHQ) synthesis
Five drops of sulfuric acid were added to a solution of 8-hydroxyquinoline-7-carboxylic acid (0.050 g, 0.26 mmol) in MeOH (5 mL).The reaction mixture was heated to reflux and stirred for 6 h. Then, thesolvent was removed in vacuum and the crude residue purified by dematellatedsilica gel chromatography (eluent: CH2Cl2/MeOH, 85:15, v/v) to afford OHQ as solid (0.054 g, quantitative).IR (ν cm-1): 1550, 1597, 2843. 1H NMR (DMSO-d6) δ ppm: 9.01(dd, J=4.8 and 1.5 Hz, 1H), 8.81 (dd, J=8.4 and 1.4 Hz, 1H), 8.0 (d,J=8.7 Hz, 1H), 7.94 (dd, J=8.4 and 4.8 Hz, 1H), 7.49 (d, J=8.72 Hz,1H), 3.37 (s, 3H). 13C NMR (DMSO-d6) δ ppm: 170.8, 158.0, 146.2,142.2, 133.9, 132.1, 128.1, 124.2, 115.7, 112.5, 52.8. HRMS m/z calcdfor C11H9NO3 [M+H]+ 204.0661; found: 204.0655.
References:
Huyen Vu, Thi;Serradji, Nawal;Seydou, Mahamadou;Brémond, éric;Ha-Duong, Nguyen-Thanh [Journal of Inorganic Biochemistry,2020,vol. 203,art. no. 110864]
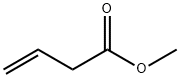
3724-55-8
89 suppliers
$121.00/1g
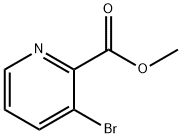
53636-56-9
146 suppliers
$10.00/1g

73776-20-2
11 suppliers
inquiry