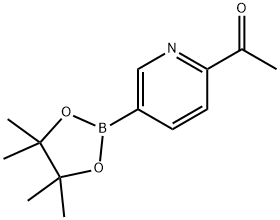
1-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl)ethanone synthesis
- Product Name:1-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl)ethanone
- CAS Number:741709-59-1
- Molecular formula:C13H18BNO3
- Molecular Weight:247.1

73183-34-3
586 suppliers
$6.00/5g
214701-49-2
200 suppliers
$24.00/1g

741709-59-1
54 suppliers
inquiry
Yield:741709-59-1 90%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane; for 4 h;Inert atmosphere;Reflux;
Steps:
Intermediate 7A: 1-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-2-yl]ethanone
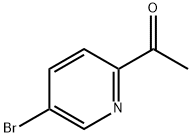
To a solution of 1-(5-bromopyridin-2-yl)ethanone (2.0 g, 9.98 mmol) in dioxane (50 mL) was added bis(pinacolato)diboron (2.8 g, 11.0 mmol), potassium acetate (2.94 g, 30.0 mmol) and [1,1 '-Bis(diphenylphosphino)ferrocene]dichloropalladium(ll) complex with dichloromethane (244 mg, 0.30 mmol) under a nitrogen atmosphere. The mixture was heated at reflux for 4 hours. The mixture was then cooled to room temperature then filtered over Celite (washing with EtOAc) and concentrated at reduced pressure. The residue was purified directly via Biotage Isolera chromatography (eluting with a gradient of eluents; 0 - 50 % EtOAc in heptane) giving the desired product (2.22 g, 90% yield) as a pale yellow solid. 1H NMR (500 MHz, DMSO-d6) δ [ppm] 8.89 (s, 1 H), 8.20 (dd, J = 7.7, 1.6 Hz, 1 H), 7.95 (d, J = 7.7 Hz, 1 H), 2.65 (s, 3H), 1.33 (s, 12H). LCMS (Analytical Method A): Rt=0.74 mins, mass ion not observed.
References:
WO2018/114786,2018,A1 Location in patent:Page/Page column 137