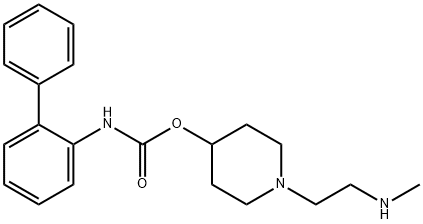
1-(2-(methylamino)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate synthesis
- Product Name:1-(2-(methylamino)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate
- CAS Number:743460-48-2
- Molecular formula:C21H27N3O2
- Molecular Weight:353.46
![1-(2-(3-((4-carbamoylpiperidin-1-yl)methyl)-N-methylbenzamido)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](/CAS/20180629/GIF/864686-28-2.gif)
864686-28-2
20 suppliers
inquiry
![1-(2-(methylamino)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](/CAS/20180703/GIF/743460-48-2.gif)
743460-48-2
54 suppliers
inquiry
Yield:743460-48-2 70%
Reaction Conditions:
with hydrogen;acetic acid;palladium 10% on activated carbon in ethanol; under 3345.86 Torr; for 3 h;
Steps:
4 Biphenyl-2-ylcarbamic Acid 1-(2-Methylaminoethyl)piperidin-4-yl Ester
Preparation 4 Biphenyl-2-ylcarbamic Acid 1-(2-Methylaminoethyl)piperidin-4-yl Ester To a Parr hydrogenation flask was added the product of Preparation 3 (40 g, 0.09 mol) and EtOH (0.5 L). The flask was flushed with nitrogen gas and palladium on activated carbon (15 g, 10 wt % (dry basis), 37% wt/wt) was added along with acetic acid (20 mL). The mixture was kept on the Parr hydrogenator under a hydrogen atmosphere (~50 psi) for 3 hours. The mixture was then filtered and washed with EtOH. The filtrate was condensed and the residue was dissolved in a minimal amount of DCM. Isopropyl acetate (10 volumes) was added slowly to form a solid which was collected to provide 22.0 g of the title compound (70% yield). MS m/z: [M+H+] calc'd for C21H27N3O2 354.2; found 354.3. Rf=2.96 min (10-70 ACN: H2O, reverse phase HPLC).
References:
US2005/203137,2005,A1 Location in patent:Page/Page column 20
![benzyl (2-(4-(([1,1'-biphenyl]-2-ylcarbamoyl)oxy)piperidin-1-yl)ethyl)(methyl)carbamate](/CAS/20180703/GIF/743460-49-3.gif)
743460-49-3
9 suppliers
inquiry
![1-(2-(methylamino)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](/CAS/20180703/GIF/743460-48-2.gif)
743460-48-2
54 suppliers
inquiry
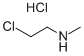
4535-90-4
101 suppliers
$14.00/1g
![piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](/CAS/20180702/GIF/171722-92-2.gif)
171722-92-2
94 suppliers
$52.00/250mg
![1-(2-(methylamino)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](/CAS/20180703/GIF/743460-48-2.gif)
743460-48-2
54 suppliers
inquiry

32315-92-7
13 suppliers
$148.00/5g
![piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](/CAS/20180702/GIF/171722-92-2.gif)
171722-92-2
94 suppliers
$52.00/250mg
![1-(2-(methylamino)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](/CAS/20180703/GIF/743460-48-2.gif)
743460-48-2
54 suppliers
inquiry
![piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](/CAS/20180702/GIF/171722-92-2.gif)
171722-92-2
94 suppliers
$52.00/250mg
![1-(2-(methylamino)ethyl)piperidin-4-yl [1,1'-biphenyl]-2-ylcarbamate](/CAS/20180703/GIF/743460-48-2.gif)
743460-48-2
54 suppliers
inquiry