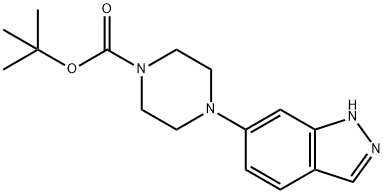
tert-Butyl 4-(1H-indazol-6-yl)piperazine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(1H-indazol-6-yl)piperazine-1-carboxylate
- CAS Number:744219-43-0
- Molecular formula:C16H22N4O2
- Molecular Weight:302.37
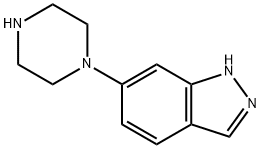
763910-07-2
23 suppliers
inquiry

24424-99-5
869 suppliers
$13.50/25G

744219-43-0
31 suppliers
$52.00/50mg
Yield:744219-43-0 66%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0 - 20; for 0.833333 h;
Steps:
C.8
8. Synthesis of tert-butyl 4-(1H-indazol-6-yl)piperazine-1-carboxylate Into a 150 mL round-bottom flask was placed a solution of 6-(piperazin-1-yl)-1H-indazole (1.67 g, 4.13 mmol) in THE (40 mL). To the mixture was added triethylamine (7 mL). This was followed by the addition of a solution of (Boc)2O (Boc anhydride?) (1.26 g, 5.78 mmol) in THF (10 mL), which was added dropwise with stirring, while cooling to a temperature of 0° C. over a time period of 20 minutes. The resulting solution was allowed to react, with stirring, for 30 minutes while the temperature was maintained at room temperature. The reaction progress was monitored by TLC (ethyl acetate/petroleum ether=1:1). The mixture was concentrated by evaporation under vacuum using a rotary evaporator. The residue was purified by eluding through a column with a 1:1 ethyl acetate/petroleum ether solvent system. This resulted in 0.83 g (66%) of tert-butyl 4-(1H-indazol-6-yl)piperazine-1-carboxylate as a grey solid. 1H NMR (300 MHz, CDCl3) ε1.48(s, 9H), 3.19-3.23(m, 4H), 3.62-3.66(m, 4H), 6.85(s, 1H), 6.95(d, 1H), 7.63(d, 1H), 7.96(s, 1H). m/z 303 [M+H]-
References:
US2008/200471,2008,A1 Location in patent:Page/Page column 34; 36
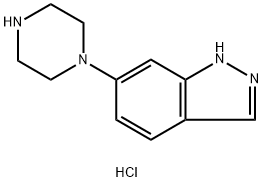
209733-16-4
0 suppliers
inquiry

24424-99-5
869 suppliers
$13.50/25G

744219-43-0
31 suppliers
$52.00/50mg
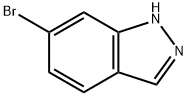
79762-54-2
377 suppliers
$3.00/1g

744219-43-0
31 suppliers
$52.00/50mg
![1H-Indazole, 6-bromo-1-[[2-(trimethylsilyl)ethoxy]methyl]-](/CAS/20200515/GIF/894808-02-7.gif)
894808-02-7
1 suppliers
inquiry

744219-43-0
31 suppliers
$52.00/50mg