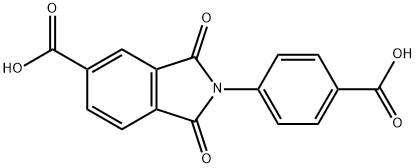
2-(4-carboxyphenyl)-1,3-dioxoisoindoline-5-carboxylic acid synthesis
- Product Name:2-(4-carboxyphenyl)-1,3-dioxoisoindoline-5-carboxylic acid
- CAS Number:7702-03-6
- Molecular formula:C16H9NO6
- Molecular Weight:311.25
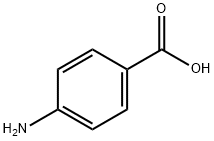
150-13-0
948 suppliers
$5.00/25g
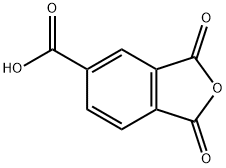
552-30-7
438 suppliers
$16.00/25g

7702-03-6
7 suppliers
$220.00/500mg
Yield:7702-03-6 84%
Reaction Conditions:
with acetic acid at 20 - 180; for 14 h;
Steps:
2.2 Synthesis of 4-carboxyphenyl-1,3-dioxoisoindoline-5-carboxylic acid (H2In-4-ba)
In a round bottle, 4-aminobenzoic acid (0.69g, 5.0mmol) in glacial acetic acid (10mL) was slowly added into a solution of trimellitic anhydride (0.96g, 5.0mmol) in glacial acetic acid (30mL), and then the solution was heated to 180°C with stirring for 8h (Scheme S1). After cooling to room temperature, the solution was stirred for further 6h and then the reaction solvents were removed under reduced pressure. The crude residue was washed with deionized water (100mL) to give H2In-4-ba as white powders. Yield 84% (1.32g, 4.2mmol). 1H NMR (300MHz, DMSO-d6): δ 8.42 (dd, J=7.5, 1.2Hz, 1H), 8.32 (d, J=0.6Hz, 1H), 8.09 (dd, J=6.8, 2.1Hz, 3H), 7.61 (dd, J=6.9, 1.8Hz, 2H) ppm. MS (EI+): m/z 311.1 [M]+ (calcd for C16H9NO6: m/z 311.25). Anal. calcd for C16H9NO6: C, 61.74; H, 2.91; N, 4.50%. Found: C, 61.93; H, 3.03; N, 4.59%. IR (KBr pellet): ν ν 1782, 1734, 1708, 1605, 1510, 1483, 1429, 1375, 1300, 1218, 1090, 927, 771, 724, 547cm-1.
References:
Chang, Chung-Yu;Tsai, Meng-Jung;Wu, Jing-Yun [Journal of Solid State Chemistry,2021,vol. 298,art. no. 122129]