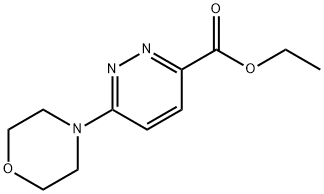
3-Pyridazinecarboxylic acid, 6-(4-morpholinyl)-, ethyl ester synthesis
- Product Name:3-Pyridazinecarboxylic acid, 6-(4-morpholinyl)-, ethyl ester
- CAS Number:77407-73-9
- Molecular formula:C11H15N3O3
- Molecular Weight:237.26

110-91-8
673 suppliers
$9.00/1g
75680-92-1
134 suppliers
$55.00/250mg

77407-73-9
4 suppliers
inquiry
Yield:77407-73-9 91%
Reaction Conditions:
with triethylamine in 1,4-dioxane at 100; for 0.5 h;Microwave irradiation;
Steps:
1
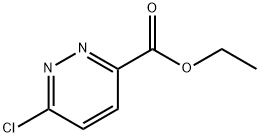
To a solution of ethyl 6-chloropyridazine-3-carboxylate (250 mg, 1.34 mmol) in 1,4-dioxane (2 mL) was added morpholine (0.14 mL, 1.61 mmol) and triethylamine (0.47 mL, 1.61 mmol). The reaction was heated in a microwave at 100°C for 30 minutes and cooled to room temperature. The reaction was partitioned between ethyl acetate (20 mL) and water (20 mL), then the layers were separated. The aqueous layer was further extracted with ethyl acetate (2 x 20 mL) and the organic layers combined, dried (MgSCb) and fdtered. The solvent was removed by evaporation to afford ethyl 6-morpholinopyridazine-3- carboxylate as an off-white solid. (0489) Yield 292 mg (91%). NMR (400 MHz, CDCh) d 7.92 (d, J=9.5 Hz, 1H), 6.86 (d, J=9.5 Hz, 1H), 4.47 (q, J=7.1 Hz, 2H), 3.86-3.84 (m, 4H), 3.79-3.75 (m, 4H), 1.44 (t, J=7.1 Hz, 3H).
References:
WO2021/252555,2021,A1 Location in patent:Paragraph 00301