
3-ThioMorpholinecarboxylic acid, 2,2-diMethyl-, (3R)- synthesis
- Product Name:3-ThioMorpholinecarboxylic acid, 2,2-diMethyl-, (3R)-
- CAS Number:774243-35-5
- Molecular formula:C7H13NO2S
- Molecular Weight:175.25
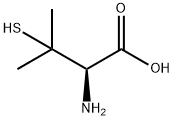
1113-41-3
222 suppliers
$17.00/100mg

107-06-2
441 suppliers
$14.00/25g

774243-35-5
23 suppliers
$240.00/100mg
Yield:-
Reaction Conditions:
with chloro-trimethyl-silane;1,8-diazabicyclo[5.4.0]undec-7-ene in DMF (N,N-dimethyl-formamide) at 0 - 25; for 20 h;
Steps:
Synthesis of benzyl ester
To a mixture of L-penicillamine (14.92 g), 1, 2-dichloroethane (300 mL) and DMF (2 mL) at 0 C was added 1, 8-diazacyclo [5. 4. 0] undec-7-ene (DBU, 22. 4 mL), followed by trimethylsilyl chloride (TMSCI, 19 mL). After stirring for 3 h, the solution was warmed to 25"C, followed by the slow addition of DBU (29.9 mL). The reaction mixture was stirred for 17 h at 25 C. Methanol (10 mL) was added and a precipitate formed. The precipitate was collected by filtration and was rinsed with a minimum amount of methanol. The solid was dried in vacuo at 50 C for 6 h to give 3 (R) -2, 2- dimethyl)-tetrahydro-2H-1,4-thiazine-3-carboxylic acid as a white powder (16 G). At 0 C, a portion of the thiazine (0.4 g, 2. 3 mmol) was dissolved in a NaOH solution (1 N, 12 mL). To the resulting mixture was added benzylchloroformate (1.4 mL, 9. 2 MMO)). After 15 h at 25 C, the solution was diluted with water (20 mL) and extracted with EtOAc (2x25 mL). The extracts were dried (MGSO4) and concentrated in vacuo. Flash column chromatography (15-20% EtOAc in hexanes) purification afforded 0.63 G of the title compound 3b3. 1H NMR (CDC13) : (mixture of two rotamers) δ 7.3 (10H, m), 7.11 (4H, br s), 4.87 and 4. 70 (1H, s), 4.40 and 4.28 (1H, d, J=6.7 Hz), 3.72 and 3.60 (1H, m), 2. 94 (1 H, m), 2.37 (1 H, t, J=3.9 Hz), 1.45 (3H, s), 1.34 (3H, s); MS (ESP) 400 (M+H+).
References:
PFIZER INC. WO2004/87720, 2004, A1 Location in patent:Page 24

79-37-8
491 suppliers
$17.67/10gm:

1113-41-3
222 suppliers
$17.00/100mg

774243-35-5
23 suppliers
$240.00/100mg

1113-41-3
222 suppliers
$17.00/100mg

774243-35-5
23 suppliers
$240.00/100mg