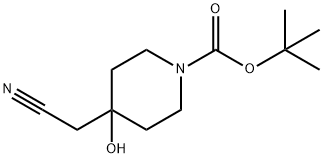
tert-Butyl 4-(cyanomethyl)-4-hydroxypiperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(cyanomethyl)-4-hydroxypiperidine-1-carboxylate
- CAS Number:774609-73-3
- Molecular formula:C12H20N2O3
- Molecular Weight:240.3

79099-07-3

75-05-8

774609-73-3
Under nitrogen protection, n-butyllithium (2.5 M hexane solution, 12 mL, 30.1 mmol) was slowly added dropwise to a pre-cooled solution of diisopropylamine (3.05 g, 30.1 mmol) in tetrahydrofuran (25 mL) at 0 °C. The reaction mixture was stirred at room temperature for 30 minutes and then cooled to -78°C. A tetrahydrofuran solution of acetonitrile (1.24 g, 30.2 mmol) was added dropwise to the reaction system at -78 °C and stirring was continued for 1 hour. Subsequently, a tetrahydrofuran (12 mL) solution of tert-butyl 4-oxopiperidine-1-carboxylate (3.0 g, 15.1 mmol) was added. The reaction mixture was warmed to room temperature and stirred for 1 hour. Upon completion of the reaction, the reaction was quenched with saturated aqueous ammonium chloride solution (100 mL) and extracted with ethyl acetate. The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate = 100:0 to 80:20) to afford N-tert-butyloxycarbonyl-4-hydroxy-4-carbonitrilemethylpiperidine (1.5 g, 41% yield) as a white solid.LC-MS (ESI): m/z [M + H]+ calculated value 241.
Yield:774609-73-3 58%
Reaction Conditions:
Stage #1: with n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78; for 1.16667 h;
Stage #2: N-tert-butyloxycarbonylpiperidin-4-one;cyanomethyl bromidewith N,N,N,N,N,N-hexamethylphosphoric triamide at 20; for 2 h;
Steps:
3.a Preparation 3; Preparation of 2,2,2-trifluoro-N-[2-(4-hydroxy-4-piperidinyl)ethyl]acetamide; a) 4-cyanomethyl-4-hydoxy-piperidine-1-carboxylic acid tert-butyl ester
To an oven-dried flask equipped with a stirring bar and rubber septum was added anhydrous THF (250 mL). Diisopropylamine (7.0 mL, 50.2 mmole) was added and the solution cooled to-78 °C. To the cooled solution was added n-butyllithium (1.6 M in hexanes, 31 mL, 50.2 MMOLE) over 10 minutes. The reaction mixture was stirred for 60 min and N-BOC-4-PIPERIDONE (10.0 g, 50.2 mmole, in 10 mL of anhydrous THF) BROMOACETONITRILE (4.2 mL, 60.2 mmole) and HMPA (20 mL) was added and the mixture stirred for an additional 2 h at RT. The reaction mixture was quenched with brine and concentrated in vacuo. The residue was taken up in ethyl acetate (500 mL) and washed with sat. NAHCO3 (2 x 200 mL). The organic layer was dried over sodium sulfate and concentrated in vacuo to obtain the title compound (7.0 g, 58%) as a yellow oil : H NMR (400 MHz, CDCl3) 8 3.90 Hz (bs, 2H), 3.16 Hz (m, 2H), 2.55 Hz (s, 2H), 1.74 Hz (m, 2H), 1.50 (m, 2H), and 1.47 (s, 9H). LC-MS (ES) M/E 241.2 (M + H).
References:
WO2004/87145,2004,A2 Location in patent:Page/Page column 31-32
