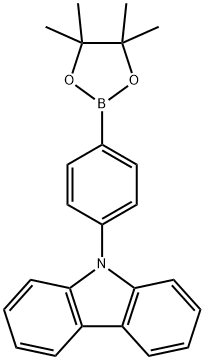
9-(4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl)-9H-carbazole synthesis
- Product Name:9-(4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl)-9H-carbazole
- CAS Number:785051-54-9
- Molecular formula:C24H24BNO2
- Molecular Weight:369.26
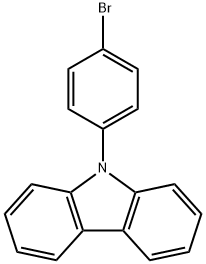
57102-42-8

73183-34-3

785051-54-9
General procedure for the synthesis of 9-[4-(4-(4-bromophenyl)carbazole and pinacol ester of bis(boronic acid)]-9H-carbazole from 9-(4-bromophenyl)carbazole and pinacol ester of bis(boronic acid): 1 g of 9-(4-bromophenyl)carbazole (3.1 mmol), 0.79 g of pinacol ester of bis(boronic acid) (3.1 mmol), 30 mL 1,4-dioxane, 0.4 g of potassium acetate (4 mmol), and 0.13 g of [1,1'-bis(diphenylbenzene)-bis(diphenyloxy)-hexahydricyclic (1,1'-bis(diphenylbenzene))]-bis(bis(diphenylbenzene)) acid (4 mmol) , 30 mL of 1,4-dioxane, 0.4 g of potassium acetate (4 mmol), and 0.13 g of [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride dichloromethane complex (0.16 mmol), and the reaction was carried out for 48 hours at 85 °C. After completion of the reaction, it was cooled to room temperature and the reaction was quenched by the addition of 200 mL of aqueous 3M hydrochloric acid. The organic layer was separated and washed with water. After the solvent was removed by evaporation, 1 g of the target product was purified by column chromatography with dichloromethane: petroleum ether = 1:2 (v/v) as eluent to give 1 g of the target product in 87% yield.

57102-42-8
279 suppliers
$5.00/250mg

73183-34-3
587 suppliers
$6.00/5g

785051-54-9
90 suppliers
$19.00/1g
Yield:785051-54-9 93%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane;water at 120; for 17 h;Inert atmosphere;
Steps:
1.3 (3) Synthesis of Compound 2A
In a nitrogen environment, 9-(4-bromophenyl)-9H-carbazole (1.5 g, 4.66 mmol), bis(pinacolato)diboron (1.54 g, 6.05 mmol), [1,1-bis(diphenylphosphineo)-ferrocene]palladium(II) dichloride (0.34 g, 0.47 mmol), and potassium acetate (1.37 g, 13.97 mmol) were added to a 1,4-dioxane/toluene (1:1) solvent and stirred. The mixed solution was stirred in an oil bath at a temperature of 120° C. for 17 hours. After completion of the reaction, the reaction temperature was cooled to room temperature, the solvent was removed, and the obtained solid was subjected to column chromatography (eluent:methylene chloride (MC) and hexane), so as to obtain 1.6 g (yield of 93%) of 9-(4-(4,4,5,5-tetramethyl-1,3,2-dioxoborolane-2-yl)phenyl)-9H-carbazole. The obtained material was identified by HRMS and HPLC analysis. High resolution mass spectrometry (matrix assisted laser desorption ionization) (HRMS (MALDI)) calcd for C24H24BNO2: m/z: 369.19 Found: 370.23.
References:
US2022/89624,2022,A1 Location in patent:Paragraph 0275; 0278-0279
61676-62-8
323 suppliers
$11.19/5G

57102-42-8
279 suppliers
$5.00/250mg

785051-54-9
90 suppliers
$19.00/1g
76-09-5
397 suppliers
$10.00/1g
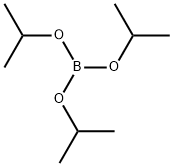
5419-55-6
388 suppliers
$12.00/5g

57102-42-8
279 suppliers
$5.00/250mg

785051-54-9
90 suppliers
$19.00/1g

73183-34-3
587 suppliers
$6.00/5g
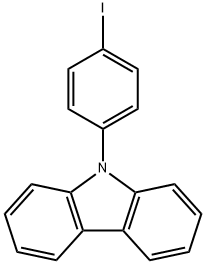
57103-15-8
89 suppliers
$20.00/100mg

785051-54-9
90 suppliers
$19.00/1g
