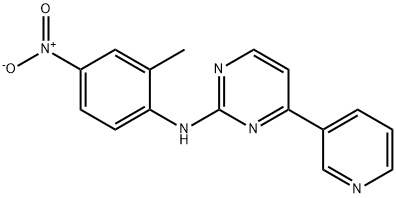
N-(2-Methyl-4-nitrophenyl)-4-(3-pyridinyl)-2-pyrimidinamine synthesis
- Product Name:N-(2-Methyl-4-nitrophenyl)-4-(3-pyridinyl)-2-pyrimidinamine
- CAS Number:796738-71-1
- Molecular formula:C16H13N5O2
- Molecular Weight:307.31
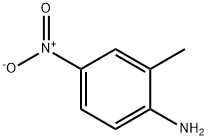
99-52-5
285 suppliers
$5.00/5G
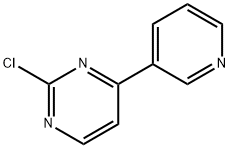
483324-01-2
89 suppliers
$65.00/10mg

796738-71-1
30 suppliers
inquiry
Yield:796738-71-1 62%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);caesium carbonate;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in 1,4-dioxane at 100; for 4 h;Inert atmosphere;
Steps:
Synthesis of N-(2-methyl-4-nitrophenyl)-4-(pyridin-3-yl)pyrimidin-2-amine, intermediate 3131-CN-I-1
To a solution under nitrogen gas of 4-(pyridin-3-yl)-2-chloropyrimidine (200 mg, 1.047 mmol) in 1,4-dioxane (10 ml) were added 2-methyl-4-nitroaniline (159 mg, 1.047mmol) and cesium carbonate (682 mg, 2.094 mmol). The resulting mixture was degassed 30 min with Argon gas, then Xantphos (61mg, 0.1047 mmol) and Pd2(dba) 3 (96 mg, 0.1047 mmol) were added and the reaction mixture was stirred at 100°C for 4 hours. The reaction mixture was then cooled to room temperature, filtered and concentrated under reduced pressure. The residue was partitioned between brine and AcOEt and the aqueous layer was extracted with AcOEt. The combined organic layers were dried over Na2SO4, filtered and concentrated under reduced pressure to give a brown oil. The crude compound was purified by flash chromatography using (silica-gel: 100-200 mesh, AcOEt -petroleum ether; 70%) to give 0.2 g (yield 62%) of a pale yellow solid corresponding to N-(2-methyl-4-nitrophenyl)-4-(pyridin-3-yl)pyrimidin-2-amine. Mass: (ES+) C16H13N5O2 required 307.11; found 308.1 [M+H], method 8.
References:
WO2017/191599,2017,A1 Location in patent:Page/Page column 196
7149-70-4
189 suppliers
$9.00/1g
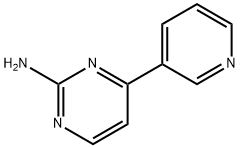
66521-66-2
208 suppliers
$13.00/250mg

796738-71-1
30 suppliers
inquiry