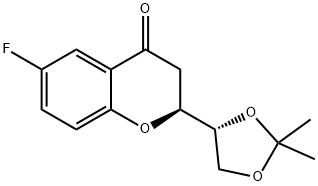
(1’R,2S)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one synthesis
- Product Name:(1’R,2S)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one
- CAS Number:797054-19-4
- Molecular formula:C14H15FO4
- Molecular Weight:266.26

797054-16-1
9 suppliers
$120.00/250mg
![(1’R,2S)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one](/CAS/20180808/GIF/797054-19-4.gif)
797054-19-4
5 suppliers
$120.00/100mg
![(1’R,2R)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one](/CAS/20180808/GIF/797054-18-3.gif)
797054-18-3
7 suppliers
$140.00/250mg
Yield:797054-18-3 40% ,797054-19-4 24%
Reaction Conditions:
with lithium hydroxide in water at 60; for 1 h;
Steps:
10 Example 10; (R,R)-2-(2',2'-dimethyl-[1,3]-dioxolane-4-yl)-6-fluoro-chroman-4-one and (S,R)-2-(2',2'-dimethyl-[1,3]-dioxolane-4-yl)-6-fluoro-chroman-4-one [X (R,R) and X (S,R)]
51.6 g (0, 19 moles) of (E)- (4R)-4, 5-ISOPROPYLYDENE-DIOXY-1- (2-hydroxy-5-fluoro-phenyl)-prop-2E-ene-1-one is suspended in water at 60°C, then 120 mg (0,005 moles) of lithium hydroxide is added to the reaction mixture and stirred at 60°C for an hour. The reaction mixture is allowed to cool to 35°C, then 200 ml of diethyl ether and 5 ml of ethanol is added to the reaction mixture. The layers are separated, the organic layer is dried, and the solvent is evaporated in vacuum. The obtained oily product is separated by, vacuum chromatography. Eluents are increasing polarity mixtures of diethyl ether and petrolether (40-70°C). Acceptable fractions are collected, evaporated. The obtained 21 g (40%) of (RR)-2- (2', 2'-DIMETHYL- [1, 3] - DIOXOLANE-4-YL)-6-FLUORO-CHROMAN-4-ONE is recrystallised from n- hexane. Mp.: 61,5-63, 50C. Optical rotation is [A] D20 (C=1, CHC13) =- 58, 1°. Continued the chromatographic process, the acceptable fractions are collected, evaporated. The obtained 12 g (24%) of (S, R)- 2- (2', 2'-DIMETHYL- [1, 3]-DIOXOLANE-4-YL)-6-FLUORO-CHROMAN-4-ONE IS recrystallised from n-hexane. MP. : 44-45°C. Optical rotation is [A] D20 (C=L, CHC13) =+57, 1°.
References:
WO2004/41805,2004,A1 Location in patent:Page 48
371-41-5
533 suppliers
$8.00/25g
![(1’R,2S)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one](/CAS/20180808/GIF/797054-19-4.gif)
797054-19-4
5 suppliers
$120.00/100mg
1707-77-3
359 suppliers
$5.00/5g
![(1’R,2S)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one](/CAS/20180808/GIF/797054-19-4.gif)
797054-19-4
5 suppliers
$120.00/100mg
394-32-1
357 suppliers
$9.00/5g
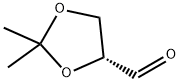
15186-48-8
322 suppliers
$8.00/5g
![(1’R,2S)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one](/CAS/20180808/GIF/797054-19-4.gif)
797054-19-4
5 suppliers
$120.00/100mg
![(1’R,2R)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one](/CAS/20180808/GIF/797054-18-3.gif)
797054-18-3
7 suppliers
$140.00/250mg
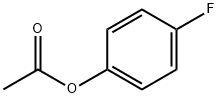
405-51-6
186 suppliers
$8.00/5g
![(1’R,2S)-2-[(1’,2’-O-Isopropylidene)dihydroxyethyl]-6-fluorochroman-4-one](/CAS/20180808/GIF/797054-19-4.gif)
797054-19-4
5 suppliers
$120.00/100mg