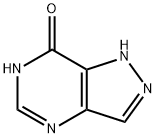
7H-PYRAZOLO[4,3-D]PYRIMIDIN-7-ONE, 1,4-DIHYDRO- synthesis
- Product Name:7H-PYRAZOLO[4,3-D]PYRIMIDIN-7-ONE, 1,4-DIHYDRO-
- CAS Number:13877-55-9
- Molecular formula:C5H4N4O
- Molecular Weight:136.11
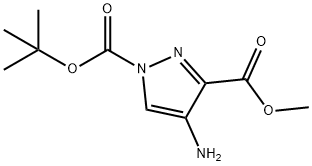
923283-63-0
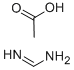
3473-63-0
![7H-PYRAZOLO[4,3-D]PYRIMIDIN-7-ONE, 1,4-DIHYDRO-](/CAS/GIF/13877-55-9.gif)
13877-55-9
GENERAL STEPS: Formamidine acetate (40.3 mmol, 4.2 g) was added to a mixed solution of Hunig base (40 mL) and n-butanol (n-BuOH, 40 mL) containing 1-tert-butyl 3-methyl 4-amino-1H-pyrazole-1,3-dicarboxylic acid (26, 8.85 g, 36.7 mmol). The reaction mixture was heated with stirring at 110 °C for 1 hour. After completion of the reaction, it was cooled to room temperature and the precipitated solid product was collected. The solid was washed with dichloromethane and then dried under reduced pressure to afford 7-hydroxypyrazolo[4,3-D]pyrimidine (27, 4.46 g, 89% yield). The structure of the product was confirmed by electrospray mass spectrometry (ES(+)MS), m/e = 137.

923283-63-0
26 suppliers
$194.40/250mg

3473-63-0
513 suppliers
$5.00/25g
![7H-PYRAZOLO[4,3-D]PYRIMIDIN-7-ONE, 1,4-DIHYDRO-](/CAS/GIF/13877-55-9.gif)
13877-55-9
98 suppliers
$28.00/100mg
Yield: 89%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in butan-1-ol at 110; for 1 h;
Steps:
17
Compound 27: Formamidine acetate (40.3 mmol, 4.2 g) was added to a solution containing 26 (8.85 g, 36.7 mmol) in Hunig's base (40 mL) and n-BuOH (40 mL). The stirred solution was heated at 110° C. for 1 hour. After cooling to ambient temperature the resulting solid was collected, washed with dichloromethane, and dried under reduced pressure to afford 27 (4.46 g, 89%), ES (+) MS m/e=137.
References:
Sunesis Pharmaceuticals, Inc. US2007/27166, 2007, A1 Location in patent:Page/Page column 84-85