
8-Bromo-1-octanol synthesis
- Product Name:8-Bromo-1-octanol
- CAS Number:50816-19-8
- Molecular formula:C8H17BrO
- Molecular Weight:209.12
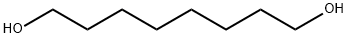
629-41-4

50816-19-8
The general procedure for the synthesis of 8-bromo-1-octanol from 1,8-octanediol was as follows: 16 g (110 mmol) of 1,8-octanediol was dissolved in 250 ml of toluene. To the solution was added 15.5 ml of hydrobromic acid (137 mmol, 1.25 eq., 48% aqueous solution), after which the reaction mixture was connected to a Dean-Stark manifold and refluxed to remove the water produced by the reaction. After 8 hours of reaction, the mixture was cooled to room temperature and washed sequentially twice with distilled water and once with brine. After drying over anhydrous sodium sulfate, the mixture was filtered and the solvent evaporated to give 8-bromo-1-octanol in quantitative yield. Nuclear magnetic resonance hydrogen spectroscopy (NMR) showed trace residues of toluene which did not affect the subsequent reaction. Densitometry (D2O calibration): 1.23 g/ml (literature value: 1.22 g/ml).13C NMR (CDCl3, ppm) data were as follows: 62.95 (CH2OH), 34.04 (BrCH2CH2), 32.78/32.71 (BrCH2 and CH2CH2OH), 29.23/28.73/ 28.09/25.65 (CH2).
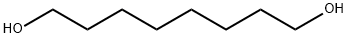
629-41-4
374 suppliers
$9.00/10g

50816-19-8
198 suppliers
$5.00/250mg
Yield:50816-19-8 100%
Reaction Conditions:
with hydrogen bromide in water;toluene for 8 h;Reflux;
Steps:
1 Step 1 : Preparation of 8-bromo-octanol
16 g (110 mmol) of octane diol have been dissolved in 250 ml toluene. After addition of 15.5 ml of HBr (137 mmol, 1.25 eq., 48% in water) the reaction mixture has been refluxed with a dean-stark receiver to remove the water from the reaction. After 8 hours the mixture was cooled to room temperature and was washed two times with distilled water and once with brine. After filtration over sodium sulfate and evaporation of the solvent the bromide was obtained in quantitative yield. In the NMR spectra, residual toluene was observed, which had no impact on the subsequent steps. D2o-c = 1.23 g/ml (lit: 1.22 g/ml) 13C NMR (CDC ; ppm): 62.95 (CH2OH), 34.04 (BrCH2CH2), 32.78/32.71 (BrCH2) and (CH2CH2OH), 29.23/28.73/28.09/25.65 (CH2)
References:
DENTSPLY DETREY GMBH;FIK, Christoph P.;KLEE, Joachim E.;SCHEUFLER, Christian;WEBER, Christoph WO2018/108948, 2018, A1 Location in patent:Page/Page column 55
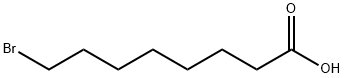
17696-11-6
322 suppliers
$13.00/1g

50816-19-8
198 suppliers
$5.00/250mg
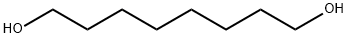
629-41-4
374 suppliers
$9.00/10g

4549-32-0
278 suppliers
$5.00/1g

50816-19-8
198 suppliers
$5.00/250mg
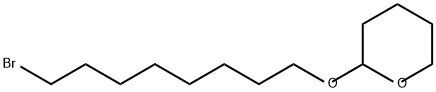
50816-20-1
50 suppliers
$42.60/1g

50816-19-8
198 suppliers
$5.00/250mg
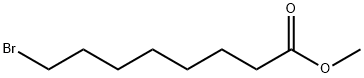
26825-92-3
52 suppliers
$220.00/1g

50816-19-8
198 suppliers
$5.00/250mg