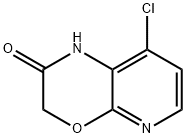
8-CHLORO-1H-PYRIDO[2,3-B][1,4]OXAZIN-2(3H)-ONE synthesis
- Product Name:8-CHLORO-1H-PYRIDO[2,3-B][1,4]OXAZIN-2(3H)-ONE
- CAS Number:1198154-62-9
- Molecular formula:C7H5ClN2O2
- Molecular Weight:184.58
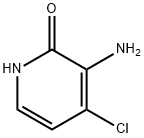
1198154-61-8
13 suppliers
$175.00/50mg

79-04-9
350 suppliers
$12.00/5g
![8-CHLORO-1H-PYRIDO[2,3-B][1,4]OXAZIN-2(3H)-ONE](/CAS/20180531/GIF/1198154-62-9.gif)
1198154-62-9
10 suppliers
$285.00/100mg
Yield:1198154-62-9 73%
Reaction Conditions:
Stage #1: 3-amino-4-chloropyridin-2-ol;chloroacetyl chloride in N,N-dimethyl-formamide at 20; for 0.5 h;Inert atmosphere;
Stage #2: with potassium carbonate in N,N-dimethyl-formamide at 20;Heating;
Steps:
5.d
d) Synthesis of 8-chloro-1H-pyrido[2,3-b][1,4]oxazin-2-one 3-amino-4-chloro-pyridin-2-ol (650 mg, 4.49 mmol) was dissolved in anhydrous N,N-dimethyl formamide (22 ml) under nitrogen atmosphere, and chloroacetyl chloride solution (0.394 ml, 4.94 mmol) was added thereto dropwise at room temperature and stirred for 30 minutes. Potassium carbonate (1.55 g, 11.24 mmol) was added thereto dropwise at room temperature, and the reaction mixture was heated to 1000 and stirred for 18 hours. The reaction mixture was cooled to room temperature and water was added to quench the reaction, and the organic layer was extracted with ethyl acetate. The combined organic layer was washed with water and saturated saline solution, dried over anhydrous sodium sulfate (Na2SO4), filtered and evaporated under reduced pressure. The residue was recrystallized from dichloromethane and diisopropyl ether to obtain white solid (608 mg, 73%). 1H-NMR (DMSO-d6, 300 MHz); δ=10.6 (s, 1H), 7.91 (d, J=5.7 Hz, 1H), 7.04 (d, J=5.4 Hz, 1H), 4.75 (s, 2H).
References:
US2011/28467,2011,A1 Location in patent:Page/Page column 53