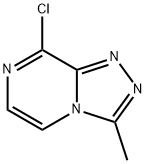
8-CHLORO-3-METHYL-[1,2,4]TRIAZOLO[4,3-A]PYRAZINE synthesis
- Product Name:8-CHLORO-3-METHYL-[1,2,4]TRIAZOLO[4,3-A]PYRAZINE
- CAS Number:68774-78-7
- Molecular formula:C6H5ClN4
- Molecular Weight:168.58
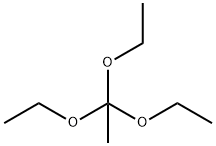
78-39-7
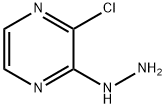
63286-28-2
![8-CHLORO-3-METHYL-[1,2,4]TRIAZOLO[4,3-A]PYRAZINE](/CAS/GIF/68774-78-7.gif)
68774-78-7
Step 2: Synthesis of 8-chloro-3-methyl-[1,2,4]triazolo[4,3-a]pyrazine [0197] To a stirred solution of 2-chloro-3-hydrazinopyrazine (2.0 g, 14 mmol) in xylene (20 mL) was slowly added triethyl orthoacetate (5.8 mL, 32 mmol). The reaction mixture was heated under reflux conditions overnight and subsequently cooled to room temperature. The precipitate precipitated was collected by filtration to afford the target compound 8-chloro-3-methyl-[1,2,4]triazolo[4,3-a]pyrazine as a brown powder (2.0 g, 87% yield). Mass Spectrometry (ESI): Calculated value C6H5ClN4 [M+H]+: 168.0; Measured value: 169.2. 1H NMR (400MHz, CDCl3) δ: 7.80 (d, J=4.8Hz, 1H), 7.71 (d, J=4.8Hz, 1H), 2.83 (s, 3H).

78-39-7
374 suppliers
$9.00/1g

63286-28-2
109 suppliers
$34.00/1g
![8-CHLORO-3-METHYL-[1,2,4]TRIAZOLO[4,3-A]PYRAZINE](/CAS/GIF/68774-78-7.gif)
68774-78-7
54 suppliers
$68.00/100mg
Yield:68774-78-7 84%
Reaction Conditions:
at 80;
Steps:
13.10 Step 10 8-chloro-3-methyl- [1,2,4] triazolo [4,3-a] pyrazine (d)
Add intermediate b (5.0 g, 0.034 mol) to triethyl orthoacetate (50 mL),The reaction was performed at 80 ° C, and the reaction time was monitored by spotting. The reaction liquid is directly filtered by cold suction,The filter cake was washed with petroleum ether, and the filter cake was dried to obtain 4.9 g of a yellow powder.The yield was 84.0%.
References:
CN110467616,2019,A Location in patent:Paragraph 0131-0134
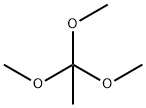
1445-45-0
358 suppliers
$10.00/5mL

63286-28-2
109 suppliers
$34.00/1g
![8-CHLORO-3-METHYL-[1,2,4]TRIAZOLO[4,3-A]PYRAZINE](/CAS/GIF/68774-78-7.gif)
68774-78-7
54 suppliers
$68.00/100mg