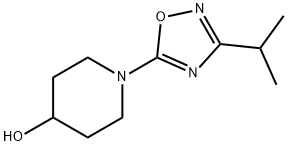
1-[3-(1-Methylethyl)-1,2,4-oxadiazol-5-yl]-4-piperidinol synthesis
- Product Name:1-[3-(1-Methylethyl)-1,2,4-oxadiazol-5-yl]-4-piperidinol
- CAS Number:832714-37-1
- Molecular formula:C10H17N3O2
- Molecular Weight:211.26
Yield:832714-37-1 71%
Reaction Conditions:
with zinc(II) chloride in diethyl ether;ethyl acetate; for 0.25 h;
Steps:
11.11.11.3
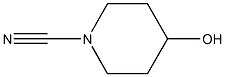
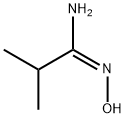
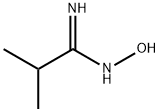
In a variation of the method described by Yarovenko et al, (Bull. Acad. Sci. USSR, DIV. Chem. Sci. 1991, 40, 1924) ZnCl2 (1 N in ether, 120 mL, 120 mmol) was added in a dropwise fashion over 15 min to a magnetically stirred solution of N-hydroxy-isobutyramidine (12.2 g, 120 mmol) and 4-HYDROXY-PIPERIDINE-L-CARBONITRILE (12.6 g, 100 mmol) in ethyl acetate (500 mL). Precipitate formed immediately upon addition, and at a point the stirring bar became immobilized in the matrix, requiring the reaction to be manually shaken for the remainder of addition. After standing for 15 min, the supernatant was decanted and filtered, and the residue was rinsed twice with ether, furnishing a hard white precipitate which was collected by filtration. This material was taken up in cone. HCl (50 mL), diluted to 4 N with ETOH (100 mL), and refluxed for 1 h. Upon cooling, a white precipitate was removed by filtration, then the filtrate was reduced to 50 ML and diluted with 100 ML water. Solid NA2CO3 was added until the mixture was basic, CH2Cl2 was added, and the resulting mixture was filtered, rinsing with CH2C12. The organic extract was separated, dried over MGS04, and the solvent was removed to afford a viscous, amber oil as 1- (3-ISOPROPYL- [1, 2,4] oxadiazol-5-yl) - piperidin-4-ol (15.0 g, 71% yield) : H NMR (CDCL3) 6 3.95 (M, 3 H), 3.37 (M, 2 H), 2.88 (M, 1 H), 2. 34 (br s, 1 H), 1.93 (m, 2 H), 1.63 (m, 2 H), 1.28 (d, 6 H, J= 7.1 Hz), MS MLZ 212.3 (M).
References:
WO2005/7647,2005,A1 Location in patent:Page/Page column 192

871681-75-3
0 suppliers
inquiry
![1-[3-(1-Methylethyl)-1,2,4-oxadiazol-5-yl]-4-piperidinol](/CAS/GIF/832714-37-1.gif)
832714-37-1
19 suppliers
inquiry
5382-16-1
0 suppliers
$9.00/5G
![1-[3-(1-Methylethyl)-1,2,4-oxadiazol-5-yl]-4-piperidinol](/CAS/GIF/832714-37-1.gif)
832714-37-1
19 suppliers
inquiry