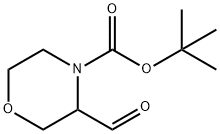
3-FORMYL-MORPHOLINE-4-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:3-FORMYL-MORPHOLINE-4-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:833474-06-9
- Molecular formula:C10H17NO4
- Molecular Weight:215.25
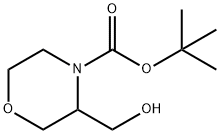
473923-56-7

833474-06-9
General procedure for the synthesis of 4-BOC-3-morpholinecarboxaldehyde from tert-butyl 3-(hydroxymethyl)morpholine-4-carboxylate: to a stirred solution of oxalyl chloride (3.73 g, 2.56 mL, 29.00 mmol) in dichloromethane (DCM, 80 mL) was slowly added at -78 °C dimethyl sulfoxide (DMSO, 4.93 g, 4.47 mL, and 63.00 mmol). after 15 min, a solution of intermediate 90 (5.70 g, 26.26 mmol) in DCM (50 mL) was added. The reaction mixture was continued to be stirred at -78 °C for 2 hours. Subsequently, triethylamine (NEt3, 13.12 g, 18.71 mL, 129.70 mmol) was added and the reaction mixture was stirred at -78 °C for 30 min, then slowly warmed to room temperature and stirring was continued for 1 hour. After completion of the reaction, the mixture was concentrated in vacuum and the residue was partitioned between water (200 mL) and ethyl acetate (EtOAc, 200 mL). The aqueous phase was extracted with EtOAc (2 x 200 mL), and the combined organic phases were washed with brine (300 mL), dried over anhydrous magnesium sulfate (MgSO4), filtered, and the solvent removed in vacuum to afford 4-BOC-3-morpholinecarboxaldehyde (4.80 g, 84% yield) as a pale yellow crude solid. Nuclear magnetic resonance hydrogen spectroscopy (1H NMR, CDCl3) data: δ 9.58 (1H, s), 4.31 (2H, m), 3.62 (2H, br.m), 3.41 (1H, br.m), 3.11 (1H, br.s), 2.93 (1H, br.m), 1.40 (9H, s).

473923-56-7
101 suppliers
$19.00/100mg

833474-06-9
61 suppliers
$137.00/100mg
Yield: 84%
Reaction Conditions:
Stage #1:tert-Butyl 3-(hydroxymethyl)-4-morpholinecarboxylate with oxalyl dichloride;dimethyl sulfoxide in dichloromethane at -78; for 2.25 h;
Stage #2: with triethylamine in dichloromethane at -78 - 20; for 1.5 h;
Steps:
91
INTERMEDIATE 91fert-Butyl 3 -formylmorpholine-4-carboxylateTo a stirred solution of oxalyl chloride (3.73 g, 2.56 mL, 29.00 mmol) in DCM (80 mL) at -780C was added DMSO (4.93 g, 4.47 mL, 63.00 mmol) and, after 15 minutes, a solution of Intermediate 90 (5.70 g, 26.26 mmol) in DCM (50 mL) was added. The reaction mixture was then stirred at -780C for a further 2 h. NEt3 (13.12 g, 18.71 mL, 129.70 mmol) was added and the reaction mixture was stirred at -780C for 30 minutes, warmed to r.t. and stirred for a further 1 h. The reaction mixture was then concentrated in vacuo and the residue partitioned between water (200 mL) and EtOAc (200 mL). The aqueous fraction was extracted with EtOAc (2 x 200 mL) and the combined organic fractions were washed with brine (300 mL), dried (MgSO4), filtered and the solvents removed in vacuo to give the title compound (4.80 g, 84%) as a pale yellow solid that was used crude. δH (CDCl3) 9.58 (IH, s), 4.31 (2H5 m), 3.62 (2H, br. m), 3.41 (IH, br. m), 3.11 (IH, br. s), 2.93 (IH, br. m), 1.40 (9H, s).
References:
UCB S.A. WO2006/114606, 2006, A1 Location in patent:Page/Page column 70
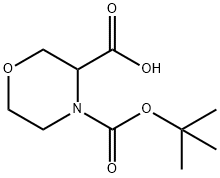
212650-43-6
162 suppliers
$46.00/250mg

833474-06-9
61 suppliers
$137.00/100mg
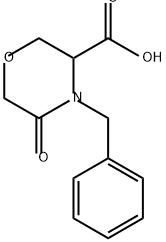
106910-79-6
88 suppliers
$50.00/250 mg

833474-06-9
61 suppliers
$137.00/100mg
110167-20-9
68 suppliers
$45.00/50mg

833474-06-9
61 suppliers
$137.00/100mg

24424-99-5
871 suppliers
$13.50/25G

833474-06-9
61 suppliers
$137.00/100mg